The dependence of the reaction rate on temperature. Van't Hoff rule. The temperature coefficient of the reaction rate and its features for biochemical processes.
Factors affecting the course of the reaction
In the human body, thousands of enzymatic reactions take place in a living cell. However, in a multistage chain of processes, the difference between the rates of individual reactions is quite large. Thus, the synthesis of protein molecules in a cell is preceded by at least two more stages: the synthesis of transfer RNA and the synthesis of ribosomes. But the time during which the concentration of tRNA molecules doubles is 1.7 minutes, protein molecules - 17 minutes, and ribosomes - 170 minutes. The rate of the overall process of the slow (limiting) stage, in our example, the rate of ribosome synthesis. The presence of a limiting reaction provides high reliability and flexibility in controlling thousands of reactions occurring in the cell. It is enough to keep under observation and regulate only the slowest of them. This method of controlling the rate of multi-stage synthesis is called the minimum principle. It allows to significantly simplify and make more reliable the system of autoregulation in the cell.
Classifications of reactions used in kinetics: reactions, homogeneous, heterogeneous and microheterogeneous; simple and complex reactions (parallel, sequential, conjugated, chain). Molecularity of the elementary act of the reaction. Kinetic equations. Reaction order. Half life

Microheterogeneous reactions -

The molecularity of the reaction is determined by the number of molecules that enter into chemical interaction in the elementary act of the reaction. On this basis, the reactions are divided into monomolecular, bimolecular and trimolecular.
Then reactions of type A -> B will be monomolecular, for example:
a) C 16 H 34 (t ° C) -> C g H 18 + C 8 H 16 - hydrocarbon cracking reaction;
b) CaC0 3 (t ° C) -> CaO + C0 2 - thermal decomposition of calcium carbonate.
Reactions like A + B -> C or 2A -> C - are bimolecular, for example:
a) C + 0 2 -> C0 2; b) 2Н 2 0 2 -> 2Н 2 0 + 0 2 etc.
Trimolecular reactions are described by general equations of the type:
a) A + B + C D; b) 2A + B D; c) 3A D.
For example: a) 2Н 2 + 0 2 2Н 2 0; b) 2NO + H 2 N 2 0 + H 2 0.
The reaction rate depending on the molecularity will be expressed by the equations: a) V = k C A - for a monomolecular reaction; b) V \u003d to C A C in or c) V \u003d to C 2 A - for a bimolecular reaction; d) V \u003d k C C in C e) V \u003d k C 2 A C in or e) V \u003d k C 3 A - for a trimolecular reaction.

Molecularity is the number of molecules that react in one elementary chemical act.



It is often difficult to establish the molecularity of a reaction, so a more formal sign is used - the order of a chemical reaction.
The order of the reaction is equal to the sum of the exponents of concentrations in the equation expressing the dependence of the reaction rate on the concentration of the reactants (the kinetic equation).
The order of the reaction most often does not coincide with the molecularity due to the fact that the reaction mechanism, i.e., the "elementary act" of the reaction (see the definition of the sign of molecularity), is difficult to establish.
Let us consider a number of examples illustrating this position.
1. The rate of dissolution of crystals is described by the equations of zero-order kinetics, despite the monomolecular nature of the reaction: AgCl (TB) -> Ag + + CI", V = k C (AgCl (TB p = k" C (AgCl (ra)) - p - density and is a constant value, i.e., the dissolution rate does not depend on the amount (concentration) of the dissolved substance.
2. The reaction of sucrose hydrolysis: CO + H 2 0 -> C 6 H 12 0 6 (glucose) + C 6 H 12 0 6 (fructose) is a bimolecular reaction, but its kinetics is described by a first-order kinetic equation: V \u003d k * C cax , since under experimental conditions, including in the body, the concentration of water is a constant value С(Н 2 0) - const.
3.  The decomposition reaction of hydrogen peroxide, proceeding with the participation of catalysts, both inorganic ions Fe 3+, Cu 2+ of metal platinum, and biological enzymes, such as catalase, has the general form:
The decomposition reaction of hydrogen peroxide, proceeding with the participation of catalysts, both inorganic ions Fe 3+, Cu 2+ of metal platinum, and biological enzymes, such as catalase, has the general form:
2H 2 0 2 -\u003e 2H 2 0 + O e, i.e., is bimolecular.
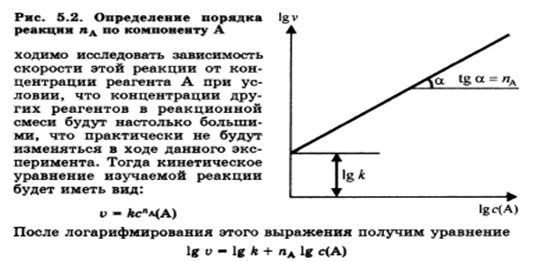
Dependence of reaction rate on concentration. Kinetic equations of reactions of the first, second and zero orders. Experimental methods for determining the rate and rate constant of reactions.






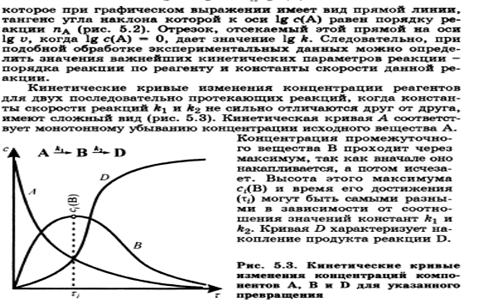
The dependence of the reaction rate on temperature. Van't Hoff rule. The temperature coefficient of the reaction rate and its features for biochemical processes.

γ is the temperature coefficient of the reaction rate.

The physical meaning of the value of γ is that it shows how many times the reaction rate changes with a change in temperature for every 10 degrees.

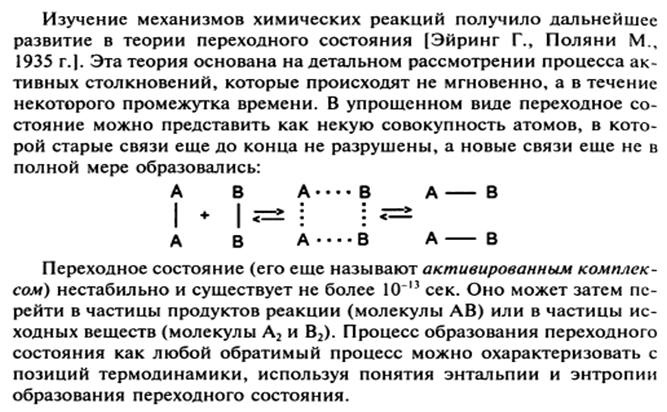
 15. The concept of the theory of active collisions. Energy profile of the reaction; activation energy; Arrhenius equation. The role of the steric factor. The concept of the theory of the transition state.
15. The concept of the theory of active collisions. Energy profile of the reaction; activation energy; Arrhenius equation. The role of the steric factor. The concept of the theory of the transition state.




The relationship of the rate constant, activation energy and temperature is described by the Arrhenius equation: k T \u003d k 0 *Ae ~ E / RT, where k t and k 0 are the rate constants at temperature T and T e e is the base of the natural logarithm, A is the steric factor.
The steric factor A determines the probability of collision of two reacting particles in the active center of the molecule. This factor is especially important for biochemical reactions with biopolymers. In acid-base reactions, the H + ion must react with the terminal carboxyl group - COO. However, not every collision of the H + ion with a protein molecule will lead to this reaction. Only those collisions that are directly carried out at certain points of the macromolecules will be effective called active centers.
It follows from the Arrhenius equation that the higher the rate constant, the lower the activation energy E and the higher the temperature T of the process.