Methodical development for independent work on the topic "types of hybridization"
STATE AUTONOMOUS EDUCATIONAL INSTITUTION
SECONDARY VOCATIONAL EDUCATION OF THE NOVOSIBIRSK REGION
"KUPINSKY MEDICAL TECHNICUM"
TOOLKIT
« »
for independent work of students
by discipline Chemistry
Section: Organic Chemistry
Topic: The subject of organic chemistry.
Theory of the structure of organic compounds
Specialty: 34.02.01 "Nursing" 1 course
Kupino
2015 academic year
Considered at the meeting
subject - cyclical methodological commission on
general education disciplines, general humanitarian and
socio - economic, mathematical
and natural science cycle
2015 Minutes
Chairman ______________ / __________________ /
Irina V. Vede
Explanatory note to the methodological manual
The manual is intended for in-depth study of the topic « Carbon hybridization types ».
Practice shows that many students find it difficult to determine the types of hybridization of carbon atoms and types of chemical bonds in the study of organic compounds.
The purpose of the manual is to help students learn to identify the types of hybridization of carbon atoms and types of chemical bonds in organic compounds.This manual is recommended for 1st year students of the specialty 34.02.01 Nursing. The manual contains theoretical material on the topic, tables for organizing knowledge, exercises for independent work and detailed answers to each of the tasks.
The manual is aimed at developing the skills of independent work with educational material, searching and using information, forming and developing creative potential, increasing interest in the discipline.
I am always ready to learn
but I don't always like
when i am taught
W. Churchill
Carbon hybridization types
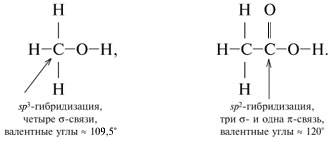
The electronic structure of the carbon atom in the ground state is 1s 2 2s 2 2p 2, on the p-orbitals of the 2nd level there are two unpaired electrons. This allows the carbon atom to form only two covalent bonds by the exchange mechanism. However, in all organic compounds, carbon forms four covalent bonds, which becomes possible as a result of hybridization of atomic orbitals.
Hybridization is the interaction of atomic orbitals with close energy values, accompanied by the formation of new "hybrid" orbitals.
Hybridization is a process that requires energy consumption, but these costs are more than offset by the energy released during the formation of a larger number of covalent bonds. the resulting "hybrid" orbitals have the shape of an asymmetric dumbbell and differ sharply from the original orbitals of the carbon atom.
Three types of hybridization are possible for a carbon atom: sp 3 -hybridization- interacting orbitals are shown with blue arrows:
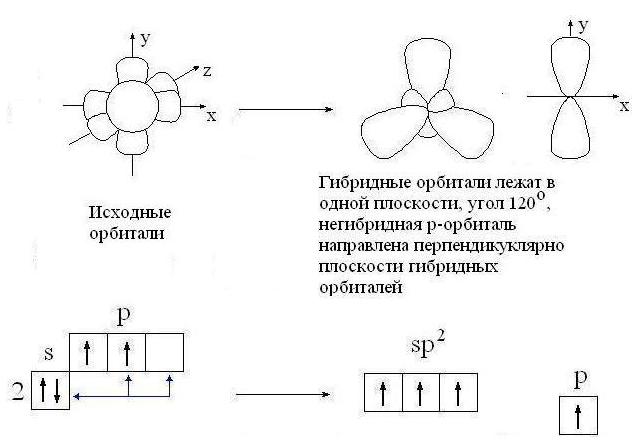
sp 2 -hybridization:
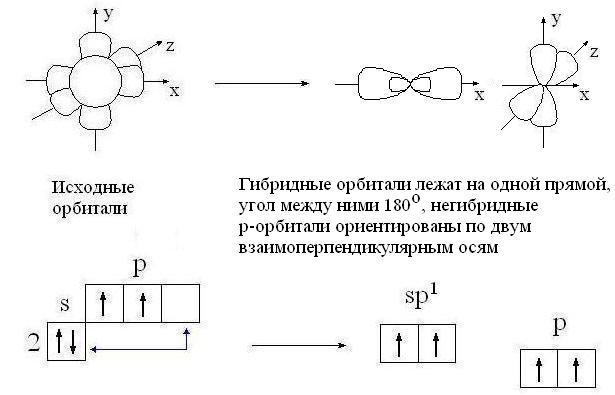
sp-hybridization:
The hybrid orbitals of the carbon atom are able to participate in the formation of only β-bonds, while the p-orbital unaffected by hybridization form only β-bonds. It is this feature that determines the spatial structure of molecules of organic substances.
Hybridization
atomic orbitals of carbon
A covalent chemical bond is formed using common bonding electron pairs of the type:
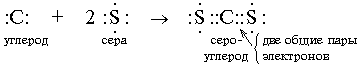
Form a chemical bond, i.e. only unpaired electrons can create a common electron pair with an "alien" electron from another atom. When writing electronic formulas, unpaired electrons are located one at a time in the orbital cell.
Atomic orbital Is a function that describes the density of the electron cloud at each point in space around the nucleus of an atom. An electron cloud is a region of space in which an electron can be found with a high probability.
To reconcile the electronic structure of the carbon atom and the valence of this element, the concept of the excitation of a carbon atom is used. In the normal (unexcited) state, a carbon atom has two unpaired 2 R 2 -electron. In an excited state (when energy is absorbed), one of 2 s 2 electrons can go to the free R-orbital. Then four unpaired electrons appear in the carbon atom:
Recall that in the electronic formula of the atom (for example, for carbon 6 С - 1 s 2 2s 2 2p 2) large numbers in front of the letters - 1, 2 - indicate the number of the energy level. Letters s and R indicate the shape of the electron cloud (orbital), and the numbers to the right above the letters indicate the number of electrons in that orbital. Everything s- spherical orbitals:
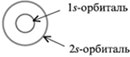
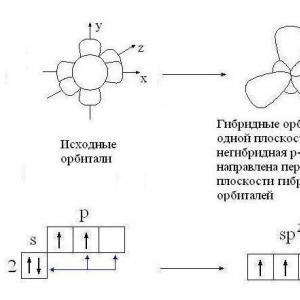
On the second energy level except 2 s-orbitals there are three 2 R-orbital. These 2 R-orbitals have an ellipsoidal shape, similar to dumbbells, and are oriented in space at an angle of 90 ° to each other. 2 R-Orbitals stand for 2 R NS , 2R y and 2 R z according to the axes along which these orbitals are located.
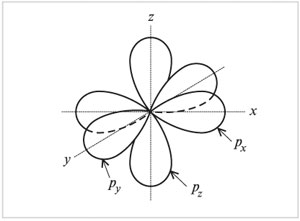
Shape and orientation
p-electron orbitals
When chemical bonds are formed, the electron orbitals acquire the same shape. So, in saturated hydrocarbons one s-orbital and three R-orbitals of a carbon atom with the formation of four identical (hybrid) sp 3-orbitals:
![]()
It - sp 3-hybridization.
Hybridization- alignment (mixing) of atomic orbitals ( s and R) with the formation of new atomic orbitals, called hybrid orbitals.
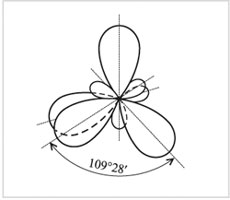
Four sp 3 -hybrid orbitals
carbon atom
Hybrid orbitals have an asymmetric shape, elongated towards the attached atom. The electron clouds repel each other and are located in space as far from each other as possible. In this case, the axes of four sp 3-hybrid orbitals turn out to be directed to the vertices of the tetrahedron (regular triangular pyramid).
Accordingly, the angles between these orbitals are tetrahedral, equal to 109 ° 28 ".
The vertices of the electron orbitals can overlap with the orbitals of other atoms. If the electron clouds overlap along the line connecting the centers of the atoms, then such a covalent bond is called sigma(
) -connection... For example, in a C 2 H 6 ethane molecule, a chemical bond is formed between two carbon atoms by overlapping two hybrid orbitals. It's a connection. In addition, each of the carbon atoms has its own three sp 3-orbitals overlap with s-orbitals of three hydrogen atoms, forming three -bonds.
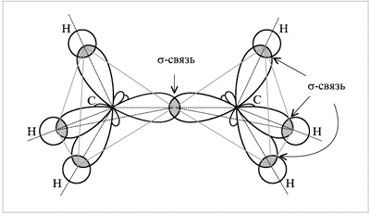
Electron cloud overlap diagram
in the ethane molecule
In total, three valence states with different types of hybridization are possible for a carbon atom. except sp 3-hybridization exists sp 2 - and sp-hybridization.
sp 2 -Hybridization- mixing one s- and two R-orbitals. As a result, three hybrid sp 2 -orbitals. These sp 2 -orbitals are located in the same plane (with the axes NS, at) and directed to the vertices of the triangle with an angle between the orbitals of 120 °. Unhybridized
R-orbital perpendicular to the plane of the three hybrid sp 2 -orbitals (oriented along the axis z). Upper half R-orbital is above the plane, the lower half is below the plane.
Type of sp 2-hybridization of carbon occurs in compounds with a double bond: C = C, C = O, C = N. Moreover, only one of the bonds between two atoms (for example, C = C) can be a bond. (The other bonding orbitals of the atom are directed in opposite directions.) The second bond is formed by overlapping non-hybrid R-orbitals on either side of the line connecting the atomic nuclei.
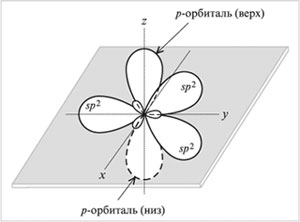
Orbitals (three sp 2 and one p)
carbon atom in sp 2 -hybridization
Covalent bond formed by lateral overlap R-orbitals of adjacent carbon atoms is called pi ( ) -connection.
Education
-connection
Due to less overlapping of the orbitals, the bond is less strong than the bond.
sp-Hybridization Is mixing (alignment in shape and energy) of one s- and one
R-orbitals with the formation of two hybrid sp-orbitals. sp-Orbitals are located on one line (at an angle of 180 °) and are directed in opposite directions from the nucleus of the carbon atom. Two
R-orbitals remain unhybridized. They are placed mutually perpendicular
directions of communication. On the image sp-orbitals are shown along the axis y, and unhybridized two
R-orbitals - along the axes NS and z.
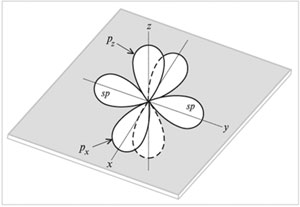
Atomic orbitals (two sp and two p)
sp-hybridized carbon
The CC triple carbon-carbon bond is composed of a β-bond arising from overlapping
sp-hybrid orbitals, and two -bonds.
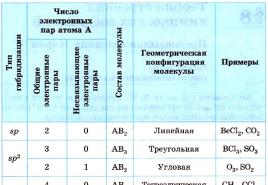
The relationship between such parameters of the carbon atom as the number of attached groups, the type of hybridization and the types of chemical bonds formed is shown in Table 4.
Covalent carbon bonds
Number of groups
related
with carbon
Type of
hybridization
Types
participating
chemical bonds
Examples of compound formulas
sp 3
Four - communication
sp 2
Three - communications and
one - bond
sp
Two - communication
and two -connections
H – CC – H
Exercises.
1. What electrons of atoms (for example, carbon or nitrogen) are called unpaired?
2. What does the concept of "common electron pairs" mean in compounds with a covalent bond (for example, CH 4 or H 2 S )?
3. What are the electronic states of atoms (for example, WITH or N ) are called basic, and which are excited?
4. What do the numbers and letters mean in the electronic formula of the atom (for example, WITH or N )?
5. What is an atomic orbital? How many orbitals on the second energy level of an atom WITH and how do they differ?
6. What is the difference between hybrid orbitals and the original orbitals from which they were formed?
7. What types of hybridization are known for a carbon atom and what are they?
Answers to exercises
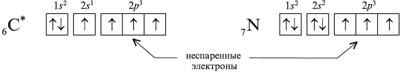
1. Electrons that are one at a time in an orbital are called unpaired electrons. For example, in the electron diffraction formula of an excited carbon atom, there are four unpaired electrons, while a nitrogen atom has three:
2. Two electrons participating in the formation of one chemical bond are called a common electron pair. Usually, before the formation of a chemical bond, one of the electrons of this pair belonged to one atom, and the other electron belonged to another atom:
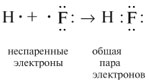
3. The electronic state of an atom, in which the order of filling of electron orbitals is observed: 1s 2, 2s 2, 2p 2, 3s 2, 3p 2, 4s 2, 3d 2, 4p 2, etc., is called the ground state. In the excited state, one of the valence electrons of the atom occupies a free orbital with a higher energy, such a transition is accompanied by the separation of paired electrons. Schematically, it is written as follows:
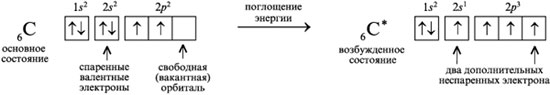
While in the ground state there were only two unpaired valence electrons, in the excited state there are four such electrons.
5.
An atomic orbital is a function that describes the density of an electron cloud at each point in space around the nucleus of a given atom. At the second energy level of the carbon atom, there are four orbitals - 2s, 2p x, 2p y, 2p z. These orbitals are different:
a) the shape of the electron cloud (s - ball, p - dumbbell);
b) p-orbitals have different orientations in space - along the mutually perpendicular axes x, y and z, they are denoted by p x, p y, p z.
6.
Hybrid orbitals differ from the original (non-hybrid) orbitals in shape and energy. For example, s-orbital is the shape of a sphere, p is a symmetric figure eight, sp-hybrid orbital is an asymmetric figure eight.
Energy Difference: E (s)< E(sр) < E(р). Таким образом, sp-орбиталь – усредненная по форме и энергии орбиталь, полученная смешиванием исходных s- и p-орбиталей.
7. Three types of hybridization are known for the carbon atom: sp 3, sp 2, and sp (see text in lesson 5).
9.
-bond - a covalent bond formed by head-on overlapping of orbitals along a line connecting the centers of atoms.
-bond - a covalent bond formed by lateral overlap of p-orbitals on both sides of the line connecting the centers of the atoms.
-Bond is shown by the second and third dash between the connected atoms.
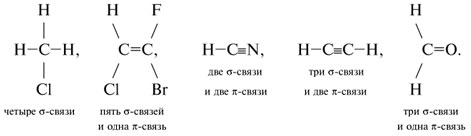
10.