§ 7. Hybridization of atomic orbitals and the geometry of molecules
The covalent bond is the most widespread in the world of organic substances, it is characterized by saturation, polarizability and directionality in space.
The saturation of a covalent bond is that the number of common electron pairs that a particular atom can form is limited. Due to this, covalent compounds have a strictly defined composition. Therefore, for example, there are H 2, N 2, CH 4 molecules, but there are no H 3, N 4, CH 5 molecules.
The polarizability of a covalent bond is the ability of molecules (and individual bonds in them) to change their polarity under the influence of an external electric field - to polarize.
As a result of polarization, non-polar molecules can become polar, and polar ones can turn into even more polar ones, up to the complete rupture of individual bonds with the formation of ions:
The directionality of the covalent bond is due to the fact that the p-, d- and f-clouds are oriented in a certain way in space. The direction of the covalent bond affects the shape of the molecules of substances, their size, interatomic distances, bond angle, i.e., the geometry of the molecules.
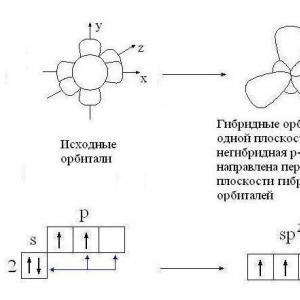
A more complete picture of the shape of molecules of organic and inorganic substances can be formed on the basis of the hypothesis of hybridization of atomic orbitals. It was proposed by L. Pauling (USA) to explain the fact of the equivalence of all chemical bonds and their symmetrical arrangement relative to the center of the CH 4, BF 3, BeCl 2 molecules, established using physical methods of studying substances. In the formation of σ-bonds, in each case, electrons in different states (s and p) should have participated from the central atom (C, B, Be), so they could not be equivalent. The theory turned out to be unable to explain the facts, a contradiction arose, which was resolved with the help of a new hypothesis. This is one of the examples showing the way of development of human cognition of the surrounding world, the possibility of ever deeper penetration into the essence of phenomena.
You got acquainted with the hypothesis of hybridization of atomic orbitals in the course of organic chemistry using the example of the carbon atom. Let us recall this one more time.
When a CH4 molecule of methane is formed, a carbon atom from the ground state passes into an excited one:
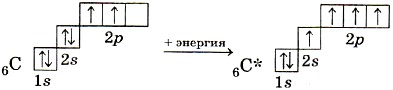
The outer electron layer of an excited carbon atom contains one s- and three unpaired p-electrons, which form four σ-bonds with four s-electrons of hydrogen atoms. It should be expected that three C - H bonds formed by pairing three p-electrons of a carbon atom with three s-electrons of three hydrogen atoms (s-p σ-bond) should differ from the fourth (ss) bond in strength , length, directionality. The study of the electron density in methane molecules shows that all bonds in its molecule are equivalent and directed to the vertices of the tetrahedron (Fig. 10). According to the hypothesis of the hybridization of atomic orbitals, the four covalent bonds of the methane molecule are formed not with the participation of "pure" s- and p-clouds of the carbon atom, but with the participation of the so-called hybrid, ie, averaged, equivalent electron clouds.
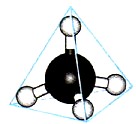
Rice. 10. Ball-and-stick model of the methane molecule
According to this model, the number of hybrid atomic orbitals is equal to the number of the original "pure" orbitals. The corresponding hybrid clouds are geometrically more advantageous than s- and p-clouds, their electron density is distributed differently, which provides a more complete overlap with s-clouds of hydrogen atoms than would be the case for "pure" s- and p-clouds.
In the methane molecule and in other alkanes, as well as in all molecules of organic compounds at the site of a single bond, the carbon atoms are in the sp 3 -hybridization state, i.e., one s- and three p-atomic clouds have undergone hybridization at the carbon atom and four identical hybrid sp 3 -atomic cloud orbitals.
As a result of the overlap of the corresponding four hybrid sp 3 -clouds of the carbon atom with the s-clouds of four hydrogen atoms, a tetrahedral methane molecule with four identical σ-bonds located at an angle of 109 ° 28 "is formed (Fig. 11).
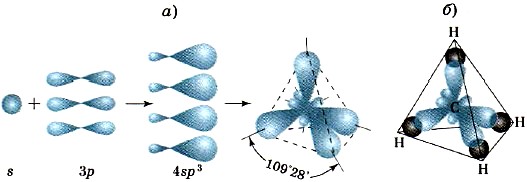
Rice. eleven.
Schemes of sp 3 -hybridization of valence electron clouds (a) and the formation of bonds in a methane molecule (b)
This type of hybridization of atoms and, consequently, the tetrahedral structure will also characterize the molecules of carbon analog compounds - silicon: SiH 4, SiCl 4.
During the formation of water and ammonia molecules, sp 3 -hybridization of the valence atomic orbitals of oxygen and nitrogen atoms also occurs. However, if at a carbon atom all four hybrid sp 3 clouds are occupied by common electron pairs, then at a nitrogen atom one sp 3 cloud is occupied by a lone electron pair, and at an oxygen atom they are already occupied by two sp 3 clouds (Fig. 12).
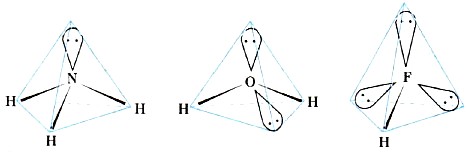
Rice. 12.
Forms of ammonia, water and hydrogen fluoride molecules
The presence of lone electron pairs leads to a decrease in bond angles (Table 8) in comparison with tetrahedral (109 ° 28 ").
Table 8
Relationship between the number of lone electron pairs and the bond angle in molecules

sp 3 -Hybridization is observed not only in atoms in complex substances, but also in atoms in simple substances. For example, atoms of such an allotropic modification of carbon as diamond.
In the molecules of some boron compounds, sp 2 -hybridization of the valence atomic orbitals of the boron atom takes place.
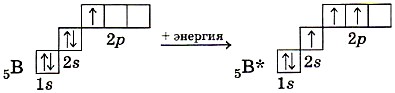
In an excited state of boron, one s and two p orbitals participate in hybridization, resulting in the formation of three sp 2 hybrid orbitals; the axes of the corresponding hybrid clouds are located in a plane at an angle of 120 ° to each other (Fig. 13).
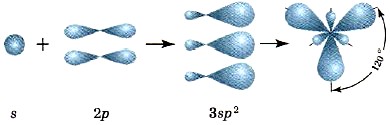
Rice. 13.
Schemes of 8p 2 -hybridization and location of sp 2 -clouds in space
Therefore, the molecules of such compounds, for example BF3, have the shape of a flat triangle (Fig. 14).
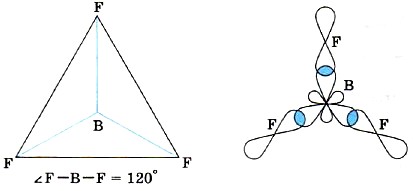
Rice. fourteen.
BF3 molecule structure
In organic compounds, as you know, sp 2 -hybridization is characteristic of carbon atoms in the molecules of alkenes at the site of the double bond, which explains the planar structure of these parts of the molecules, as well as molecules of dienes and arenes. sp 2 -Hybridization is also observed at carbon atoms and in such an allotropic modification of carbon as graphite.
In the molecules of some beryllium compounds, sp-hybridization of the valence orbitals of the beryllium atom in an excited state is observed.
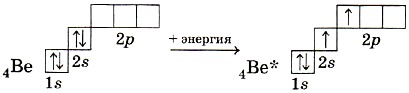
Two hybrid clouds are oriented relative to each other at an angle of 180 ° (Fig. 15), and therefore the beryllium chloride molecule BeCl 2 has a linear shape.
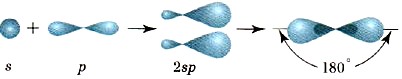
Rice. 15.
Sp-hybridization schemes and the location of sp-clouds in space
A similar type of hybridization of atomic orbitals exists for carbon atoms in alkynes - hydrocarbons of the acetylene series - at the site of the triple bond.
Such hybridization of orbitals is characteristic of carbon atoms in another of its allotropic modifications - carbyne:
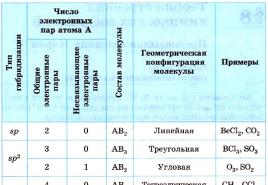
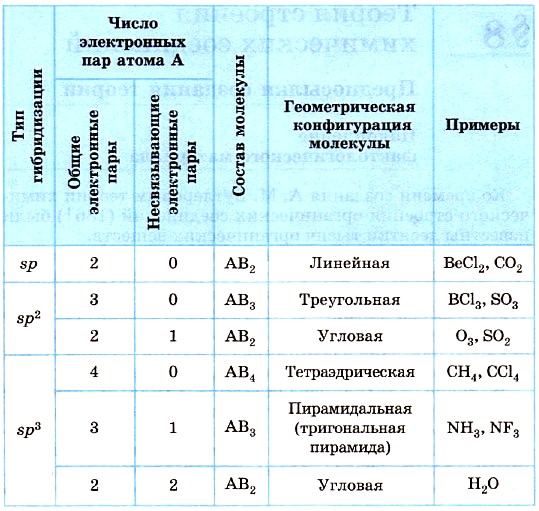
Table 9 shows the types of geometric configurations of molecules corresponding to some types of hybridization of the orbitals of the central atom A, taking into account the influence of the number of free (non-bonding) electron pairs.
Table 9
Geometric configurations of molecules corresponding to various types of hybridization of the outer electron orbitals of the central atom
Questions and tasks for § 7
- In the molecules of hydrogen compounds of carbon, nitrogen and oxygen, the formulas of which are CH 4, NH 3 and H 2 O, the valence orbitals of the central atoms of non-metals are in the sp 3 -hybridization state, but the bond angles are different - 109 ° 28 "107 ° 30" and 104 ° 27 ", respectively. How can this be explained?
- Why is graphite electrically conductive and diamond not?
- What geometric shape will the molecules of two fluorides have - boron and nitrogen (BF 3 and NF 3, respectively)? Give a reasoned answer.
- The silicon fluoride molecule SiF 4 has a tetrahedral structure, and the bromine chloride ВСl 3 molecule has the shape of a triangle - planar. Why?