Lesson 26. Obtaining hydrogen and its use
In lesson 26 "" from the course " Chemistry for dummies»Learn about the production of hydrogen in laboratories and in industry, as well as find out in which industries it is used.
Hydrogen is widely used in engineering and laboratory research. The world industrial production of hydrogen is measured in tens of millions of tons per year.
The choice of an industrial method for obtaining simple substances depends on the form in which the corresponding element is found in nature. Hydrogen is found in nature mainly in compounds with atoms of other elements. Therefore, to obtain it, it is necessary to use chemical methods. The same methods are used to obtain hydrogen in laboratory practice.
In laboratories, hydrogen is obtained by the method you already know, acting with acids on metals: iron, zinc, etc. Place three zinc granules on the bottom of the test tube and add a small volume of hydrochloric acid. Where the acid comes into contact with zinc (on the surface of the granules), bubbles of colorless gas appear, which quickly rise to the surface of the solution:
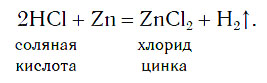
Zinc atoms replace hydrogen atoms in acid molecules, as a result of which a simple substance hydrogen H 2 is formed, the bubbles of which are released from the solution. To obtain hydrogen in this way, you can use not only hydrochloric acid and zinc, but also some other acids and metals.
We collect hydrogen by air displacement, placing the tube upside down (explain why), or by water displacement and test it for cleanliness. We tilt the test tube with the collected hydrogen to the flame of the spirit lamp. A dull clap indicates that the hydrogen is pure; The loud "barking" sound of an explosion indicates that it is contaminated with an air admixture.
In chemical laboratories, to obtain relatively small volumes of hydrogen, the method of decomposing water using an electric current is usually used:
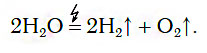
From the equation of the decomposition process, it follows that 2 mol of hydrogen and 1 mol of oxygen are formed from 2 mol of water. Consequently, the ratio of the volumes of these gases is also equal to:
![]()
Production of hydrogen in industry
Obviously, given the huge volumes of industrial production, readily available and cheap substances should be the raw material for hydrogen production. These substances are natural gas (CH 4 methane) and water. Natural gas reserves are very large, and water is practically unlimited.
The cheapest way to produce hydrogen is by decomposition of methane when heated:
![]()
This reaction is carried out at a temperature of about 1000 ° C.
In industry, hydrogen is also obtained by passing water vapor over hot coal:
![]()
There are other industrial methods for producing hydrogen.
Application of hydrogen
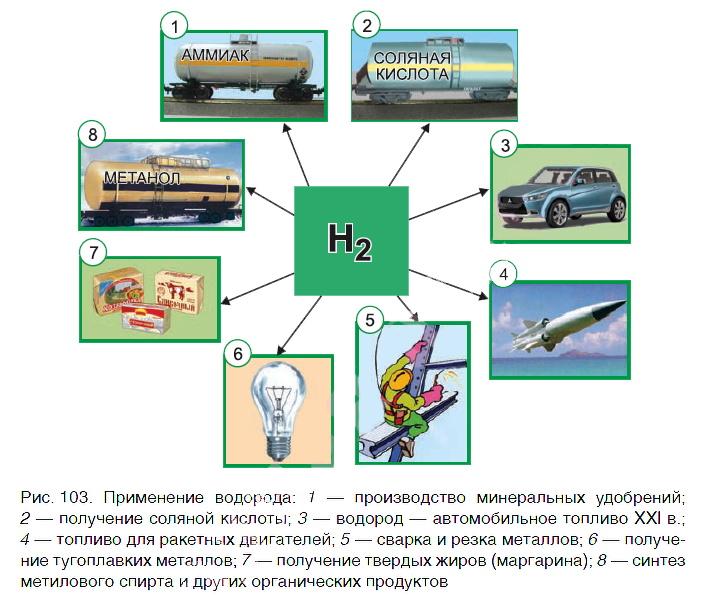
Hydrogen is widely used in practice. The main areas of its industrial use are shown in Figure 103.
A significant part of the hydrogen is used for oil refining. About 25% of the produced hydrogen is consumed for the synthesis of ammonia NH 3. It is one of the most important products of the chemical industry. The production of ammonia and nitrogen fertilizers on its basis is carried out in our country at JSC "Grodno Azot". The Republic of Belarus supplies nitrogen fertilizers to many countries of the world.
A large amount of hydrogen is consumed to obtain hydrochloric acid. The reaction of combustion of hydrogen in
oxygen is used in rocket engines that launch aircraft into space. Hydrogen is also used to obtain metals from oxides. In this way, refractory metals molybdenum and tungsten are obtained.
In the food industry, hydrogen is used in the production of margarine from vegetable oils. The reaction of combustion of hydrogen in oxygen is used for welding. If you use special burners, you can increase the flame temperature to 4000 ° C. At this temperature, welding is carried out with the most refractory materials.
Currently, in a number of countries, including Belarus, research has begun on replacing non-renewable energy sources (oil, gas, coal) with hydrogen. When hydrogen burns in oxygen, an environmentally friendly product is formed - water. And carbon dioxide, which causes the greenhouse effect (warming the environment), is not emitted.
It is believed that since the middle of the XXI century. serial production of hydrogen cars should be started. Household fuel cells, whose work is also based on the oxidation of hydrogen with oxygen, will find wide application.
Lesson summary:
- In the laboratory, hydrogen is produced by the action of acids on metals.
- In industry, for the production of hydrogen, available and cheap raw materials are used - natural gas, water.
- Hydrogen is a promising energy source for the 21st century.
Hopefully lesson 26 " Hydrogen production and its application”Was understandable and informative. If you have any questions, write them in the comments. If there are no questions, then proceed to the next lesson.