Solving problems on calculating the amount of heat required to heat a body or emitted by it during cooling.
Plan-synopsis
open physics lesson in 8 "E" class
MOU gymnasium No. 77, about. Tolyatti
physics teachers
Ivanova Maria Konstantinovna
Lesson topic:
Solving problems on calculating the amount of heat required to heat a body or emitted by it during cooling.
The date of the:
The purpose of the lesson:
to work out practical skills in calculating the amount of heat required for heating and released during cooling;
develop counting skills, improve logical skills when analyzing the plot of problems, solving high-quality and calculation problems;
to develop the ability to work in pairs, respect the opinion of the opponent and defend their point of view, to be accurate in the design of problems in physics.
Lesson equipment:
computer, projector, presentation on the topic (Appendix # 1), materials from a single collection of digital educational resources.
Lesson type:
solving problems.
“Stick your finger into the flame of a match, and you will experience a sensation that has no equal in heaven or on earth; however, everything that has happened is simply a consequence of the collisions of molecules. "
J. Wheeler
During the classes:
Organizing time
Greetings from students.
Checking for absent students.
Communication of the topic and objectives of the lesson.
Homework check.
1.Frontal poll
What is called the specific heat of a substance? (Slide number 1)
What is the unit of the specific heat capacity of a substance?
Why do bodies of water freeze slowly? Why doesn't the ice leave rivers and especially lakes for a long time, although the weather has been warm for a long time?
Why is it warm enough on the Black Sea coast of the Caucasus even in winter?
Why do many metals cool much faster than water? (Slide number 2)
2. Individual survey (cards with multilevel assignments for several students)
Learning a new topic.
1. Repetition of the concept of the amount of heat.
Quantity of heat- a quantitative measure of the change in internal energy during heat exchange.
The amount of heat absorbed by the body is considered to be positive, and the amount released is negative. The expression “the body has a certain amount of heat” or “the body contains (stored) a certain amount of heat” does not make sense. The amount of heat can be received or given away in any process, but it cannot be possessed.
During heat exchange at the interface between bodies, the interaction of slowly moving molecules of a cold body with rapidly moving molecules of a hot body occurs. As a result, the kinetic energies of the molecules are leveled and the velocities of the molecules of the cold body increase, and that of the hot body decreases.
During heat exchange, there is no transformation of energy from one form to another; part of the internal energy of a hot body is transferred to a cold body.
2. The formula for the amount of heat.
Let's derive a working formula to solve problems of calculating the amount of heat: Q = cm ( t 2 - t 1 ) - writing on the board and in notebooks.
We find out that the amount of heat given off or received by the body depends on the initial temperature of the body, its mass and on its specific heat capacity.
In practice, thermal calculations are often used. For example, when constructing buildings, it is necessary to take into account how much heat the entire heating system must give to the building. You should also know how much heat will go into the surrounding space through windows, walls, doors.
3 . Dependence of the amount of heat on various quantities . (Slides No. 3, No. 4, No. 5, No. 6)
4 . Specific heat (Slide number 7)
5. Units for measuring the amount of heat (Slide number 8)
6. An example of solving a problem for calculating the amount of heat (Slide number 10)
7. Solving problems for calculating the amount of heat on the board and in notebooks


We also find out that if heat exchange occurs between bodies, then the internal energy of all heating bodies increases by as much as the internal energy of cooling bodies decreases. For this, we will use an example of a solved problem from § 9 of the textbook.

Dynamic pause.
IV. Consolidation of the studied material.
1. Questions for self-control (Slide number 9)
2. Solving quality problems:
Why is it hot in the deserts during the day, and at night the temperature drops below 0 ° C? (The sand has a low specific heat, so it quickly heats up and cools.)
A piece of lead and a piece of steel of the same mass was hit with a hammer the same number of times. Which piece is hotter? Why? (The piece of lead is heated more, because the specific heat of lead is less.)
Why iron stoves heat a room rather than brick stoves, but do not stay warm for so long? (The specific heat of copper is less than that of brick.)
Copper and steel weights of the same mass were given equal amounts of heat. Which weight will change the temperature more? (Copper, tk. the specific heat of copper is less.)
What is more energy spent on heating water or heating an aluminum pan if their masses are the same? (For heating water, since the specific heat capacity of water is large.)
As you know, iron has a higher specific heat than copper. Consequently, the sting of a burner made of iron would have a greater supply of internal energy than the same sting made of copper, if their masses and temperatures were equal. Why, despite this, is the soldering iron tip made of copper? (Copper has great thermal conductivity.)
It is known that the thermal conductivity of metal is much higher than the thermal conductivity of glass. Why, then, are calorimeters made of metal and not glass? (The metal has a high thermal conductivity and low specific heat, due to which the temperature inside the calorimeter quickly levels out, and little heat is spent on heating it. In addition, radiation from metal is significantly less than radiation from glass, which reduces heat loss.)
It is known that loose snow protects the soil well from freezing, because it contains a lot of air, which is a poor conductor of heat. But after all, layers of air adjoin the soil not covered with snow. Why, then, does it not freeze too much? (The air, in contact with the soil uncovered with snow, is in motion all the time, is mixed. This moving air removes heat from the earth and intensifies the evaporation of moisture from it. The air between the snow particles is inactive and, like a poor conductor of heat, protects the ground from freezing.)
3. Solving computational problems
The first two tasks are solved by highly motivated students at the blackboard with a collective discussion. We find the right approaches in reasoning and formulating problem solutions.
Problem number 1.
When a piece of copper was heated from 20 ° C to 170 ° C, 140,000 J of heat was consumed. Determine the mass of copper.
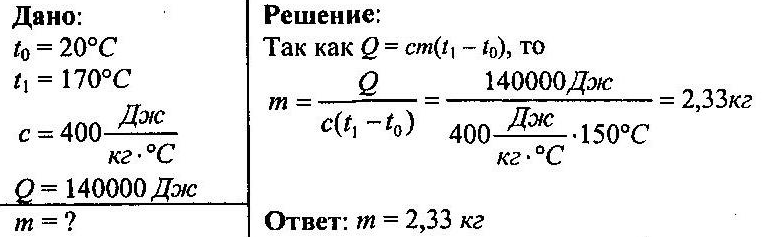
Problem number 2
What is the specific heat capacity of a liquid if it took 150,000 J to heat 2 liters of it at 20 ° C. The density of the liquid is 1.5 g / cm³
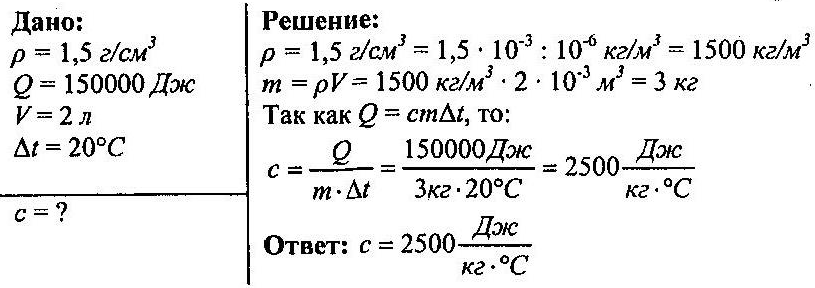
Students find answers to the following problems in pairs:
Problem number 3.
Two copper balls with masses m o and 4m o are heated so that both balls receive the same amount of heat. At the same time, the large ball warms up by 5 ° C. How hot is the ball of the smaller mass?
Problem number 4.
How much heat is released when 4 m³ of ice is cooled from 10 ° C to –40 ° C?
Problem number 5.
In which case is more heat required to heat two substances if the heating of two substances is the same ∆ t 1 = ∆t 2 The first substance is a brick of mass 2 kg and c = 880J / kg ∙ ° C, and brass is a mass of 2 kg and c = 400 J / kg ∙ ° C
Problem number 6.
A steel bar with a mass of 4 kg was heated. In this case, 200,000 J of heat was consumed. Determine the final body temperature if the initial temperature is t 0 = 10 ° C
When students independently solve problems, it is natural that questions arise. We analyze the most frequently asked questions collectively. For those questions that are of a private nature, individual answers are given.
Reflection. Marking.
Teacher: So guys, what did you learn in the lesson today and what did you learn new?
Sample student responses :
We have worked out the skills of solving high-quality and design problems on the topic “Calculation of the amount of heat required for heating the body and released during cooling”.
We have seen in practice how subjects such as physics and mathematics overlap and relate.
Home assignment:
Solve problems # 1024, 1025, from the collection of problems by V.I. Lukashik, E.V. Ivanova.
Independently come up with a problem to calculate the amount of heat required to heat a body or emitted by it during cooling.