Examples of solving typical tasks. How many times will the reaction rate increase:
Example 1
How many times will the reaction rate increase:
a) C + 2 H 2 = CH 4
b) 2 NO + Cl 2 = 2 NOCl
when the pressure in the system triples?
Solution:
A threefold increase in pressure in the system is equivalent to a threefold increase in the concentration of each of the gaseous components.
In accordance with the law of mass action, we write down the kinetic equations for each reaction.
a) Carbon is a solid phase, and hydrogen is a gaseous phase. The rate of a heterogeneous reaction does not depend on the concentration of the solid phase; therefore, it is not included in the kinetic equation. The rate of the first reaction is described by the equation
Let the initial hydrogen concentration be X, then v 1 = kx 2. After increasing the pressure three times, the hydrogen concentration became 3 X, and the reaction rate v 2 = k (3x) 2 = 9kx 2. Next, we find the ratio of the speeds:
v 1: v 2 = 9kx 2: kx 2 = 9.
So, the reaction speed will increase 9 times.
b) The kinetic equation of the second reaction, which is homogeneous, can be written as 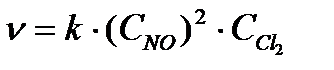 ... Let the initial concentration NO is equal to X, and the initial concentration Сl 2 is equal to at, then v 1 = kx 2 y; v 2 = k (3x) 2 3y = 27kx 2 y;
... Let the initial concentration NO is equal to X, and the initial concentration Сl 2 is equal to at, then v 1 = kx 2 y; v 2 = k (3x) 2 3y = 27kx 2 y;
v 2:v 1 = 27.
The reaction speed will increase 27 times.
Example 2
The reaction between substances A and B proceeds according to the equation 2A + B = C. The concentration of substance A is 6 mol / l, and substance B is 5 mol / l. The reaction rate constant is 0.5 (l 2 ∙ mol -2 ∙ s –1). Calculate the rate of the chemical reaction at the initial moment and at the moment when 45% of substance B remains in the reaction mixture.
Solution:
Based on the law of effective masses, the rate of the chemical reaction at the initial moment is equal to:
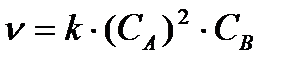 = 0.5 ∙ 6 2 ∙ 5 = 90.0 mol ∙ s -1 ∙ l -1
= 0.5 ∙ 6 2 ∙ 5 = 90.0 mol ∙ s -1 ∙ l -1
After some time, 45% of substance B will remain in the reaction mixture, that is, the concentration of substance B will become equal to 5. 0.45 = 2.25 mol / l. This means that the concentration of substance B has decreased by 5.0 - 2.25 = 2.75 mol / l.
Since substances A and B interact with each other in a ratio of 2: 1, the concentration of substance A decreased by 5.5 mol / l (2.75 ∙ 2 = 5.5) and became equal to 0.5 mol / l (6, 0 - 5.5 = 0.5).
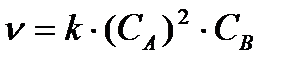 = 0.5 (0.5) 2 ∙ 2.25 = 0.28 mol ∙ s -1 ∙ l -1.
= 0.5 (0.5) 2 ∙ 2.25 = 0.28 mol ∙ s -1 ∙ l -1.
Answer: 0.28 mol ∙ s -1 ∙ l -1
Example 3
Temperature Coefficient of Reaction Rate g is 2.8. How many degrees was the temperature increased if the reaction time was reduced 124 times?
Solution:
According to the Van't Hoff rule v 1 = v 2 ×... Reaction time t is a quantity inversely proportional to the speed, then v 2 / v 1 = t 1 / t 2 = 124.
t 1 / t 2 = = 124
Let's take the logarithm of the last expression:
lg ( )= lg 124;
DТ / 10× lgg = lg 124;
DT = 10× lg124 / lg2.8 » 47 0 .
The temperature was increased by 47 0.
Example 4
With an increase in temperature from 10 0 С to 40 0 С, the reaction rate increased by 8 times. What is the value of the activation energy of the reaction?
Solution:
The ratio of the reaction rates at different temperatures is equal to the ratio of the rate constants at the same temperatures and is equal to 8. In accordance with the Arrhenius equation
k 2 / k 1 = A × / A = 8
Since the preexponential factor and activation energy are practically independent of temperature, then
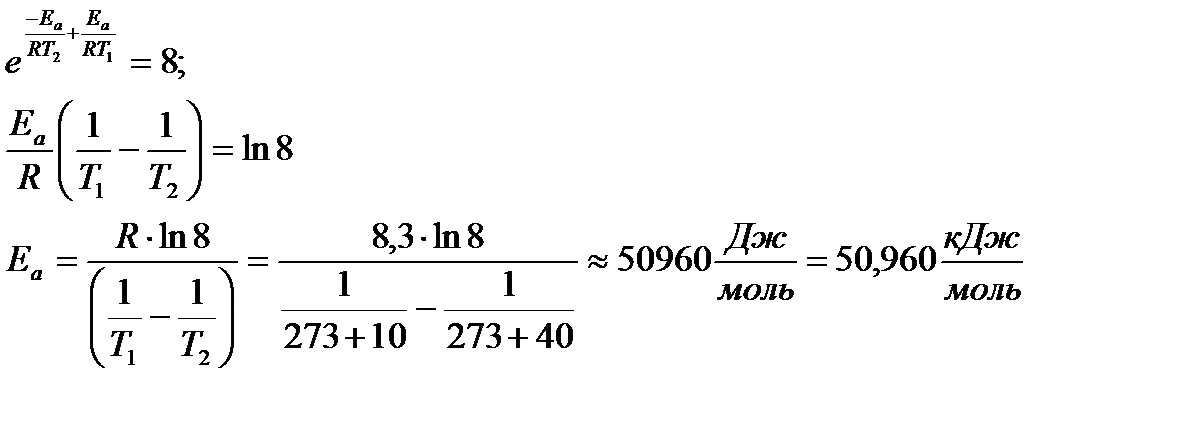
Example 5
At a temperature of 973 TO equilibrium constant of the reaction
NiO + H 2 = Ni + H 2 O (g)
Solution:
We assume that the initial concentration of water vapor was zero. The expression for the equilibrium constant of this heterogeneous reaction is as follows: 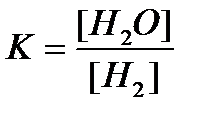 .
.
Let by the moment of equilibrium the concentration of water vapor has become equal to x mol / l. Then, in accordance with the stoichiometry of the reaction, the hydrogen concentration decreased by x mol / l and became equal (3 - x) mol / L.
Substitute the equilibrium concentrations in the expression for the equilibrium constant and find X:
K = x / (3 - x); x / (3 - x) = 0.32; x = 0.73 mol / l.
So, the equilibrium concentration of water vapor is 0.73 mol / l, the equilibrium concentration of hydrogen is 3 - 0.73 = 2.27 mol / l.
Example 6
How will the reaction affect the balance? 2SO 2 + O 2 ⇄ 2 SO 3; DH = -172.38 kJ:
1) increased concentration SO 2, 2) increase in pressure in the system,
3) cooling of the system, 4) introduction of a catalyst into the system?
Solution:
According to Le Chatelier's principle, with increasing concentration SO 2 the equilibrium will shift towards the process that leads to the expenditure SO 2, that is, towards the direct reaction of formation SO 3.
The reaction comes with a change in number mole gaseous substances, therefore, a change in pressure will lead to a shift in equilibrium. With an increase in pressure, the equilibrium will shift towards a process that counteracts this change, that is, going with a decrease in the number mole gaseous substances, and, consequently, with a decrease in pressure. According to the reaction equation, the number mole gaseous starting materials is equal to three, and the number mole the products of the direct reaction is equal to two. Therefore, with increasing pressure, the equilibrium will shift towards the direct reaction of formation SO 3.
Because DН< 0, then the direct reaction proceeds with the release of heat (exothermic reaction). The reverse reaction will proceed with heat absorption (endothermic reaction). In accordance with Le Chatelier's principle, cooling will cause the equilibrium to shift towards a reaction proceeding with the release of heat, that is, towards a direct reaction.
The introduction of a catalyst into the system does not cause a shift in the chemical equilibrium.
Example 7
At 10 0 C, the reaction ends in 95 s, and at 20 0 C in 60 s. Calculate the activation energy for this reaction.
Solution:
The reaction time is inversely proportional to its speed. Then 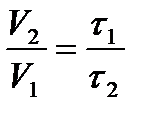 .
.
The relationship between the reaction rate constant and the activation energy is determined by the Arrhenius equation:
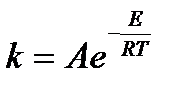
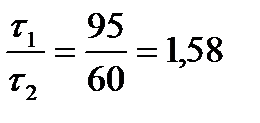
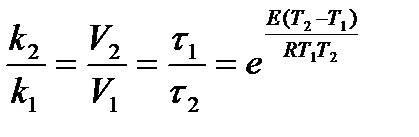 = 1,58.
= 1,58.
ln1.58 = 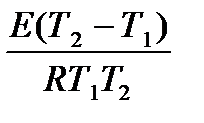 ;
;
Answer: 31.49 kJ / mol.
Example 8
In the synthesis of ammonia N 2 + 3H 2 2NH 3, equilibrium was established at the following concentrations of reactants (mol / l):
Calculate the equilibrium constant for this reaction and the initial concentrations of nitrogen and hydrogen.
Solution:
Determine the equilibrium constant KC of this reaction:
K C= 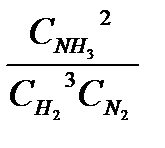 = (3,6) 2 / 2,5 (1,8) 3 = 0,89
= (3,6) 2 / 2,5 (1,8) 3 = 0,89
The initial concentrations of nitrogen and hydrogen are found on the basis of the reaction equation. For the formation of 2 mol NH 3, 1 mol of nitrogen is consumed, and the formation of 3.6 mol of ammonia required 3.6 / 2 = 1.8 mol of nitrogen. Taking into account the equilibrium concentration of nitrogen, we find its initial concentration:
C ref (H 2) = 2.5 + 1.8 = 4.3 mol / l
For the formation of 2 mol of NH 3, it is necessary to consume 3 mol of hydrogen, and to obtain 3.6 mol of ammonia, 3 ∙ 3.6: 2 = 5.4 mol is required.
C ref (H 2) = 1.8 + 5.4 = 7.2 mol / l.
Thus, the reaction started at concentrations (mol / l): C (N 2) = 4.3 mol / l; C (H 2) = 7.2 mol / l
List of tasks for topic 3
1. The reaction proceeds according to the scheme 2A + 3B = C. The concentration of A decreased by 0.1 mol / L. How did the concentrations of substances B and C change?
2. The initial concentrations of substances participating in the reaction CO + H 2 O = CO 2 + H 2 were equal (mol / l, left to right): 0.3; 0.4; 0.4; 0.05. What are the concentrations of all substances at the moment when ½ part of the initial CO concentration has reacted?
3. How many times will the reaction rate 2A + B change C, if the concentration of substance A is increased by 2 times, and the concentration of substance B is reduced by 3?
4. Some time after the start of the reaction 3A + B 2C + D concentration of substances were (mol / l, from left to right): 0.03; 0.01; 0.008. What are the initial concentrations of substances A and B?
5. In the CO + Cl 2 system COCl 2, the concentration of CO was increased from 0.03 to 0.12 mol / L, and chlorine from 0.02 to 0.06 mol / L. How many times has the speed of the direct reaction increased?
6. How many times should the concentration of substance B in the 2A + B system be increased? A 2 B, so that when the concentration of substance A decreases by 4 times, the speed of the direct reaction does not change?
7. How many times should the concentration of carbon monoxide (II) in the 2CO system be increased? CO 2 + C, so that the reaction rate increases 100 times? How will the reaction rate change with a 5-fold increase in pressure?
8. How long will it take to complete the reaction at 18 0 С, if at 90 0 С it is completed in 20 seconds, and the temperature coefficient of the reaction rate γ = 3.2?
9. At 10 0 С the reaction ends in 95 s, and at 20 0 С in 60 s. Calculate the activation energy.
10. How many times will the reaction rate increase when the temperature rises from 30 0 to 50 0 C, if the activation energy is 125.5 kJ / mol?
11. What is the value of the activation energy of the reaction, the rate of which at 300 K is 10 times higher than at 280 K?
12. What is the activation energy of a reaction if its rate doubles as the temperature rises from 290 to 300 K?
13. The activation energy of some reaction is 100 kJ / mol. How many times will the reaction rate change when the temperature rises from 27 to 37 0 С?
14. The initial concentrations of substances participating in the reaction N 2 + 3H 2 = 2NH 3 are equal (mol / l, from left to right): 0.2; 0.3; 0. What are the concentrations of nitrogen and hydrogen at the moment when the concentration of ammonia becomes equal to 0.1 mol / l.
15. How many times will the reaction rate 2A + B change C, if the concentration of substance A is increased by 3 times, and the concentration of substance B is reduced by 2 times?
16. Initial concentrations of substances A and B in the reaction A + 2B C were 0.03 and 0.05 mol / L, respectively. The reaction rate constant is 0.4. Find the initial reaction rate and the rate after a certain time, when the concentration of substance A decreases by 0.01 mol / L.
17. How will the reaction rate 2NO + O 2 change? 2NO 2 if: a) increase the pressure in the system by 3 times; b) reduce the volume of the system by 3 times?
18. How many times will the rate of the reaction occurring at 298 K increase if the activation energy is reduced by 4 kJ / mol?
19. At what temperature the reaction will complete in 45 minutes, if at 293 K it takes 3 hours? The temperature coefficient of reaction is 3.2.
20. The activation energy of the reaction NO 2 = NO + 1 / 2O 2 is equal to 103.5 kJ / mol. The rate constant of this reaction at 298K is 2.03 ∙ 10 4 s -1. Calculate the rate constant of this reaction at 288 K.
21. The reaction CO + Cl 2 COCl 2 takes place in a volume of 10 liters. Equilibrium mixture composition: 14 g CO; 35.6 g Cl 2 and 49.5 g COCl 2. Calculate the equilibrium constant of the reaction.
22. Find the equilibrium constant of the reaction N 2 O 4 2NO 2, if the initial concentration of N 2 O 4 is 0.08 mol / l, and by the time equilibrium is reached, 50% of N 2 O 4 has been dissociated.
23. The equilibrium constant of the reaction A + B C + D is equal to one. Initial concentration [A] o = 0.02 mol / l. How many percent of A is transformed if the initial concentrations of B, C and D are 0.02; 0.01 and 0.02 mol / L, respectively?
24. For the reaction Н 2 + Вr 2 2HBr at a certain temperature K = 1. Determine the composition of the equilibrium mixture if the initial mixture consisted of 3 mol of H 2 and 2 mol of bromine.
25. After mixing gases A and B in the system A + B C + D, equilibrium is established at the following concentrations (mol / l): [B] = 0.05; [C] = 0.02. The equilibrium constant of the reaction is 4 ∙ 10 3. Find the initial concentrations of A and B.
26. The equilibrium constant of the reaction A + B C + D is equal to one. Initial concentration [A] = 0.02 mol / l. How many percent of A is transformed if the initial concentration [B] is 0.02; 0.1 and 0.2 mol / L?
27. At the initial moment of the reaction ammonia synthesis, the concentrations were (mol / l): = 1.5; = 2.5; = 0. What is the concentration of nitrogen and hydrogen at an ammonia concentration of 0.15 mol / l?
28. Equilibrium in the H 2 + I 2 2HI system was established at the following concentrations (mol / l): = 0.025; = 0.005; = 0.09. Determine the initial concentrations of iodine and hydrogen if there was no HI at the initial moment of the reaction.
29. When a mixture of carbon dioxide and hydrogen was heated in a closed vessel, an equilibrium was established CO 2 + H 2 CO + H 2 O. The equilibrium constant at a certain temperature is 1. How many percent of CO 2 will turn into CO if you mix 2 mol of CO 2 and 1 mol H 2 at the same temperature.
30. The equilibrium constant of the reaction FeO + CO Fe + CO 2 at a certain temperature is equal to 0.5. Find the equilibrium concentrations of CO and CO 2 if the initial concentrations of these substances were 0.05 and 0.01 mol / L, respectively.
Solutions
Theoretical explanations
The concentration of a solution is the relative content of a solute in a solution. There are two ways to express the concentration of solutions - fractional and concentration.
Fractional method
Mass fraction of a substance ω - dimensionless value or expressed as a percentage, calculated by the formula
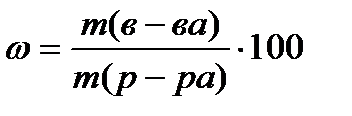 %, (4.1.1)
%, (4.1.1)
where m (in-va)- the mass of the substance, G;
m (solution)- the mass of the solution, G.
Mole fraction χ
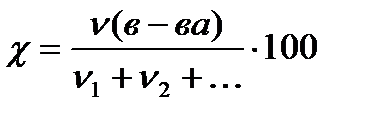 %, (4.1.2)
%, (4.1.2)
where ν (in-va)- the amount of substance, mole;
ν 1+ν 2+ ... is the sum of the amounts of all substances in the solution, including the solvent, mole.
Volume fraction φ - the quantity is dimensionless or expressed as a percentage, calculated by the formula
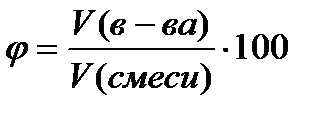 %, (4.1.3)
%, (4.1.3)
where V (in-va)- the volume of the substance, l;
V (mixtures)- the volume of the mixture, l.
Concentration method
Molar concentration C M , mol / L, calculated by the formula
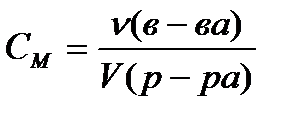 , (4.1.4)
, (4.1.4)
where ν (in-va)- the amount of substance, mole;
V (solution)- the volume of the solution, l.
Abbreviated designation 0.1 M means 0.1 molar solution (concentration 0.1 mol / l).
Normal concentration S N , mol / L, calculated by the formula
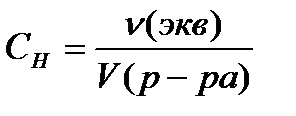 or
or 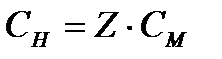 , (4.1.5)
, (4.1.5)
where ν (equiv)- the amount of substance equivalent, mole;
V (solution)- the volume of the solution, l;
Z Is an equivalent number.
Abbreviated designation 0.1n. means 0.1 normal solution (concentration 0.1 mol eq / l).
Molar concentration C b , mol / kg, calculated by the formula
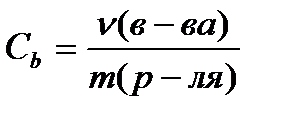 (4.1.6)
(4.1.6)
where ν (in-va)- the amount of substance, mole;
m (p-la)- the mass of the solvent, kg.
Titer T , g / ml, calculated by the formula
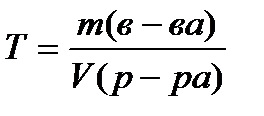 (4.1.7)
(4.1.7)
where m (in-va)- the mass of the substance, G;
V (solution)- the volume of the solution, ml.
Let us consider the properties of dilute solutions, which depend on the number of particles of the solute and on the amount of solvent, but practically do not depend on the nature of the dissolved particles (colligative properties ) .
These properties include: lowering the pressure of the saturated vapor of the solvent over the solution, increasing the boiling point, lowering the freezing point of the solution compared to a pure solvent, osmosis.
Osmosis is a one-way diffusion of substances from solutions through a semipermeable membrane separating a solution and a pure solvent or two solutions of different concentrations.
In a solvent-solution system, solvent molecules can move through the baffle in both directions. But the number of solvent molecules passing into solution per unit time is greater than the number of molecules moving from solution to solvent. As a result, the solvent enters a more concentrated solution through a semipermeable membrane, diluting it.
The pressure that must be applied to a more concentrated solution so that the solvent stops entering it is called osmotic pressure .
Solutions characterized by the same osmotic pressure are called isotonic .
Osmotic pressure is calculated using the Van't Hoff formula
where ν - the amount of substance, mole;
R- gas constant equal to 8.314 J / (mol K);
T- absolute temperature, TO;
V- the volume of the solution, m 3;
WITH- molar concentration, mol / l.
According to Raoul's law, the relative decrease in the saturated vapor pressure over the solution is equal to the molar fraction of the dissolved non-volatile substance:
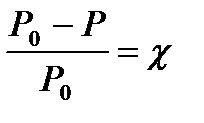 (4.1.9)
(4.1.9)
An increase in the boiling point and a decrease in the freezing point of solutions in comparison with a pure solvent, as a consequence of Raoult's law, are directly proportional to the molar concentration of the solute:
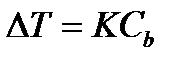 (4.1.10)
(4.1.10)
where is the temperature change;
Molar concentration, mol / kg;
TO- the proportionality coefficient, in the case of an increase in the boiling point, is called the ebulioscopic constant, and to lower the freezing point, it is called cryoscopic.
These constants, numerically different for the same solvent, characterize an increase in the boiling point and a decrease in the freezing point of a one-molar solution, i.e. when dissolving 1 mol of non-volatile electrolyte in 1 kg of solvent. Therefore, they are often referred to as a molal increase in the boiling point and a decrease in the freezing point of a solution.
Cryoscopic and ebulioscopic constants do not depend on the nature of the solute, but depend on the nature of the solvent and are characterized by the dimension 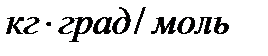 .
.
Table 4.1.1 - Cryoscopic K K and ebulioscopic K E constants for some solvents
Cryoscopy and ebulioscopy- methods for determining certain characteristics of substances, for example, the molecular weights of solutes. These methods make it possible to determine the molecular weight of substances nondissociating upon dissolution by lowering the freezing point and increasing the boiling point of solutions of known concentration:
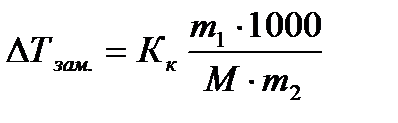 (4.1.11)
(4.1.11)
where is the mass of the solute in grams;
Solvent weight in grams;
Molar mass of solute in g / mol;
1000 - conversion factor from grams of solvent to kilograms.
Then the molar mass of the non-electrolyte is determined by the formula
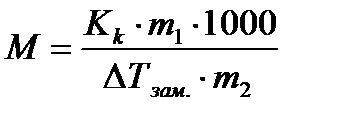 (4.1.12)
(4.1.12)
Solubility S shows how many grams of a substance can dissolve in 100 g of water at a given temperature. The solubility of solids, as a rule, increases with increasing temperature, and for gaseous substances it decreases.
Solids have a wide variety of solubilities. Along with soluble substances, there are slightly soluble and practically insoluble in water. However, there are no absolutely insoluble substances in nature.
In a saturated solution of a poorly soluble electrolyte, a heterogeneous equilibrium is established between the precipitate and the ions in the solution:
A m B n 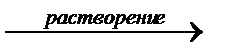 mA n + + nB m -.
mA n + + nB m -.
sediment  saturated solution
saturated solution
In a saturated solution, the rates of dissolution and crystallization are the same , and the concentrations of ions over the solid phase are equilibrium at a given temperature.
The equilibrium constant of this heterogeneous process is determined only by the product of the activities of ions in solution and does not depend on the activity of the solid component. She got the name solubility product PR .
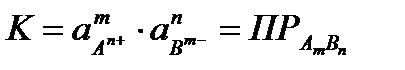 (4.1.13)
(4.1.13)
Thus, the product of the activities of ions in a saturated solution of a poorly soluble electrolyte at a given temperature is a constant value.
If the electrolyte has a very low solubility, then the concentration of ions in its solution is negligible. In this case, the interionic interaction can be neglected and the concentration of ions can be considered equal to their activities. Then the product of solubility can be expressed in terms of the equilibrium molar concentration of electrolyte ions:
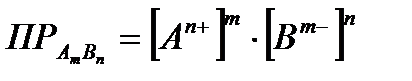 . (4.1.14)
. (4.1.14)
The solubility product, like any equilibrium constant, depends on the nature of the electrolyte and on temperature, but does not depend on the concentration of ions in the solution.
With an increase in the concentration of one of the ions in a saturated solution of a poorly soluble electrolyte, for example, as a result of the introduction of another electrolyte containing the same ion, the product of the concentration of ions becomes greater than the value of the product of solubility. In this case, the equilibrium between the solid phase and the solution shifts towards the formation of a precipitate. The precipitate will form until a new equilibrium is established, at which condition (4.1.14) is again satisfied, but with different ratios of ion concentrations. With an increase in the concentration of one of the ions in a saturated solution over the solid phase, the concentration of the other ion decreases so that the solubility product remains constant under unchanged conditions.
So, the condition for precipitation is:
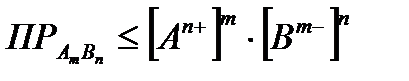 . (4.1.15)
. (4.1.15)
If the concentration of any of its ions is reduced in a saturated solution of a poorly soluble electrolyte, then ETC the product of ion concentrations becomes larger. The equilibrium will shift towards the dissolution of the precipitate. Dissolution will continue until condition (4.1.14) is satisfied again.