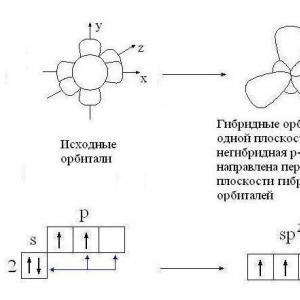
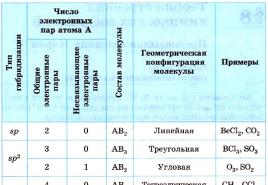
What types are reactions divided into according to ...
Questions:
1 ... What types of reactions are divided on the basis of "composition and number of reagents and reaction products"? Give examples of reaction equations of each type.
2 ... What are compound reactions? With what thermal effect do they proceed? Give examples of compound reaction equations.
3 ... What are decomposition reactions? With what thermal effect do they proceed? Give examples of decomposition reaction equations.
4 ... What are the characteristics of substitution reactions? Write down a block of equations for substitution reactions characterizing the properties of halogens.
5 ... Formulate Berthollet's rule. Illustrate it with the reaction equations: a) for formic acid; b) for sulfuric acid.
6 ... Write down the thermochemical equation of the methane combustion reaction if it is known that the combustion of 5.6 liters of this gas (n.u.) releases 225 kJ of heat.
7 ... When 18 g of aluminum are combined in oxygen, 547 kJ of heat is released. Write a thermochemical equation for this reaction.
8 ... The combustion of 7 g of ethylene releases 350 kJ of heat. Determine the thermal effect of the reaction.
9 ... Thermochemical equation for the reaction of complete combustion of acetylene:
2C2H2 + 5O2-> 4CO2 + 2H2O + 2610 kJ.
Calculate the amount of heat that is released during the combustion of 1.12 L of acetylene (n.u.).
Answers: