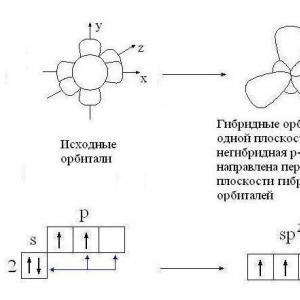
Methods for producing hydrogen
The relevance of this issue today is quite high due to the fact that the scope of using hydrogen is extremely extensive, and in its pure form it is practically not found anywhere in nature. That is why several techniques have been developed that allow the extraction of this gas from other compounds through chemical and physical reactions. This is discussed in the article above.
Methods for producing hydrogen in industrial conditions
Extraction by methane conversion... Water in a vapor state, preheated to 1000 degrees Celsius, is mixed with methane under pressure and in the presence of a catalyst. This method is interesting and proven, it should also be noted that it is constantly being improved: the search for new catalysts, cheaper and more effective, is underway.
Consider the most ancient method of producing hydrogen - coal gasification... Provided there is no air access and a temperature of 1300 degrees Celsius, coal and water vapor are heated. Thus, hydrogen is displaced from water, and carbon dioxide is obtained (hydrogen will be at the top, carbon dioxide, also obtained as a result of the reaction, is at the bottom). This will be the separation of the gas mixture, everything is very simple.
Obtaining hydrogen by electrolysis of water is considered the simplest option. For its implementation, it is necessary to pour a soda solution into the container, and also place two electrical elements there. One will be charged positively (anode) and the other negatively (cathode). When current is applied, hydrogen will go to the cathode and oxygen to the anode.
Electrolysis of WaterObtaining hydrogen by the method partial oxidation... For this, an alloy of aluminum and gallium is used. It is placed in water, which leads to the formation of hydrogen and alumina during the reaction. Gallium is necessary for the reaction to take place in full (this element will prevent aluminum from oxidizing prematurely).
Recently acquired relevance method of using biotechnology: under the condition of a lack of oxygen and sulfur, chlamydomonas begin to intensively release hydrogen. A very interesting effect that is now being actively studied.
 Chlamydomonas
Chlamydomonas
Do not forget another old, proven method of hydrogen production, which consists in using different alkaline elements and water. In principle, this technique is feasible in a laboratory setting with the necessary safety measures in place. Thus, in the course of the reaction (it proceeds with heating and with catalysts), a metal oxide and hydrogen are formed. It remains only to collect it.
Get hydrogen by interaction of water and carbon monoxide possible only in an industrial environment. Carbon dioxide and hydrogen are formed, the principle of their separation is described above.
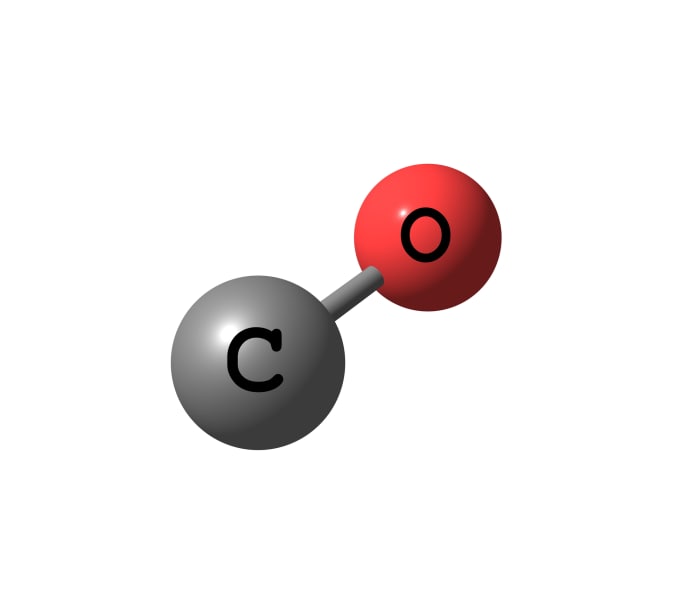 Carbon monoxide
Carbon monoxide
Is it possible to get hydrogen in the laboratory or at home?
This can be done, but it's better not. The reason for this is the explosiveness of hydrogen. In addition, all reactions with its release are exothermic, that is, they are accompanied by intense heat release. In the event that you decide to synthesize hydrogen at home and are not going to deviate from your intentions, then you will need to do this on the street. If an emergency occurs, there will be fewer casualties. In the best case, you will only get off with burns from the heat that will arise during the chemical reaction.
In order to produce hydrogen at home, several reagents are used: copper sulfate, kitchen salt, aluminum and water. The process itself includes several stages.
- It is necessary to mix a solution of vitriol with a solution of sodium chloride, resulting in a green solution.
- Place aluminum in the prepared solution.
- The bubbles that have accumulated around aluminum are nothing more than hydrogen. When the aluminum foil is covered with a red coating, this will indicate that the copper has been completely displaced by the aluminum from the solution.
Again, if you decide to work on producing hydrogen at home, then you need to make sure that others do not suffer as a result of your activities. shows what interesting and safe experiments with hydrogen can be done at home.