Covalent bond. Molecular structure
6.2.1. Determination of the type of chemical bond by difference
electronegativities of the atoms forming the bond
The formation of various types of chemical bonds depends on the ability of atoms to donate or attract electrons. This ability is characterized by the magnitude of electronegativity (EO). The larger the EO value. the greater the ability of atoms to attract electrons. If the difference in EO (ΔEO) between the two atoms forming the bond is zero (ΔEO = 0), then such a bond is non-polar covalent. At 0< ΔЭО < 2 связь полярная ковалентная. Ионная связь образуется при Δ ЭО > 2.
Example 3. Determine what type of bond is in the compounds KC1, H 2, SO 2.
Solution. Using the EO values for each atom (Appendix, Table A4), we find ΔEO.
The most polar is the Na – H bond, since in this case the ΔEO value is the largest.
6.2.2. Finding the electric moment of a bond dipole and a molecule
To estimate the polarity of the bond and the molecule, the electric moment of the dipole μ is used, which is equal to the product of the dipole length and the electric charge q (q= 1.602 10 -19 C), i.e. μ = 1 q... For polar bonds and molecules μ> 0, for nonpolar ones μ = 0. The electric moment of a dipole - a system of two equal and opposite electric charges in sign - is a vector quantity directed from a positive to a negative charge. Unit of measurement μ - Debye ( D): D= 3.33 · 10 –30 Kl · m. The electric moment of the dipole of a diatomic molecule is equal to the electric moment of the bond dipole. The electric moment of the dipole of a polyatomic molecule is equal to the vector sum of the electric moments of the dipoles of all bonds.
Example 5. Determine the electric moment of the dipole of the HF molecule and its direction if μ of the bond is 1.9 D(Appendix, Table A5).
Solution. The HF molecule is diatomic, has a linear structure: H – F. Consequently, the electric moment of the bond dipole is equal to the electric moment of the dipole of the molecule (1.91 D) and is directed from hydrogen, which has a positive charge, to negative fluorine: H®F (EO H = 2.1; EO F = 4.0).
Example 6. The BeН 2 molecule has a linear structure. The bond angle Н – Ве – Н is 180 °. The Be – H bonds are polar (EO Be = 1.5; EO H = 2.1). The Н 2 О molecule has an angular structure (the Н – О – Н bond angle is 104 ° 30 "). The Н – О bonds are polar (EO H = 2.1; EO O = 3.5). Will both molecules be polar?
Solution. In the BeH 2 molecule, the bonds are polar and the vector of the electric moment of the Be – H 2 bond dipole is directed from (+) to (-); from beryllium with a lower EO to hydrogen with a higher EO, namely H. The vector sum of the electrical moments of the bond dipoles, equal in magnitude and opposite in sign, is equal to zero. Therefore, the molecule is non-polar (= 0).
In the Н 2 О molecule, the polar Н – О bonds are located at an angle of 104 ° 30 "(Fig. 1). Therefore, their electric moments of the bond dipoles are not mutuallyRice. 1. Electric moment of the dipole
molecules Н 2 О
Solution. Electric charge q= 1.602 · 10 –19 C. The electric moment of the dipole of the molecule is found by the formula
μ = 1 q= 1.602 · 10 -19 · 3.37 · 10 -11 = 5.4 · 10 -30 C · m. (1.63 D).
Answer: 5.4 · 10 –30 C · m. = (1.63 D).
6.2.3. Explanation of the structure of molecules by the method of valence bonds (BC)
The formation of molecules by the VS method can occur by an exchange or donor-acceptor mechanism. The first is based on overlapping one-electron atomic orbitals; the second is associated with the presence of donor atoms, which have an electron pair, and acceptor atoms, which have free atomic orbitals.
Example 8. Explain the formation of chemical bonds in the NH 3 molecule and its structure by the exchange mechanism.
Solution . To explain the formation of chemical bonds in the NH 3 molecule by the exchange mechanism, it is necessary to write the electronic formulas of nitrogen and hydrogen atoms; determine their valence electrons and the covalence of each atom.
Nitrogen 7 N 1 s 2 2s 2 2R 3 – Rs 2 2R 3. The covalence of an atom is three 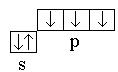 ... Therefore, three covalent bonds are formed.
... Therefore, three covalent bonds are formed.
Hydrogen 1 H 1 s 1 – ss 1 ; covalence is 1, the atom forms one covalent bond.
In the formation of three chemical bonds N – H in the NH 3 molecule, three one-electron R-orbitals of the nitrogen atom and one one-electron s-orbital of each of the three hydrogen atoms. Overlapping s-p orbitals occurs in the direction of greatest elongation R-orbitals in space located at an angle of 90 °. After overlapping, the shape of the NH 3 molecule is a trigonal pyramid with an H – N – H bond angle equal to 107 ° 20 "(Fig. 2).
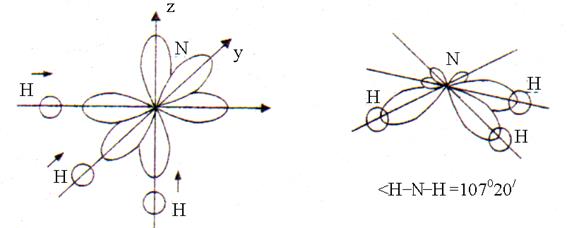
Rice. 2. The structure of the ammonia molecule
The deviation of the angle from 90 ° is due to the fact that the N – H bond is polar (EO N = 3.0; EO H = 2.1). Bonding electron pairs are slightly shifted from hydrogen atoms to nitrogen atoms (EO N> EO H). Therefore, the positively charged hydrogen atoms, as well as the electron pairs of the three N – H bonds, repel each other, which leads to an increase in the bond angle to 107 ° 20 ".
Example 9. Explain the formation of the ammonium ion NH 4 + from the ammonia molecule NH 3 and the hydrogen ion H + by the donor-acceptor mechanism.
Solution. The formation of bonds in the NH 3 molecule by the exchange mechanism is given in example 8. As follows from the explanations, three covalent bonds are formed by the exchange mechanism due to three valence one-electron R-orbitals of the nitrogen atom. But, in addition, the nitrogen atom also has a two-electron valence s-orbital (2 s 2), which can be a donor in relation to an acceptor - a hydrogen ion with a free orbital. Therefore, the nitrogen atom can form the fourth covalent bond by the donor-acceptor mechanism. The formation of the fourth covalent bond (donor-acceptor) in the ammonium ion NH 4 + can be represented by the following scheme:
Donor Acceptor
The ammonium ion has the form of a tetrahedron due to s 1 R 3 -hybridization (see clause 6.2.4).
6.2.4. Determination of the type of hybridization of atomic orbitals
and the spatial configuration of the molecule by the VS method
Example 10. Determine the type of hybridization of atomic orbitals, the spatial configuration and polarity of the BH 3 molecule.
Solution.
Bor: 5 V l s 2 2s 2 2p 1 – R-element; valence electrons 2 s 2 2p 1 ; the covalence of an atom in the normal (unexcited) state is equal to one 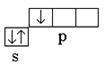 .
.
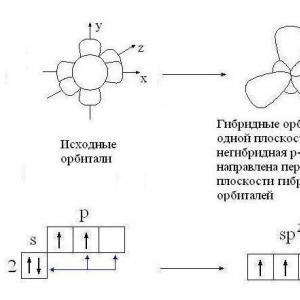
Hydrogen 1 H 1 s 1 – s-element; valence electrons 1 s 1 ; covalence is equal to one. In the normal state, a boron atom can form only one chemical bond due to one unpaired R-electron. However, the composition of the BH 3 molecule indicates that boron forms three chemical bonds, i.e. its covalency is three. This is possible when an atom is transferred from a normal to an excited state (Fig. 3).
Rice. 3. Formation of hybrid orbitals of the boron atom
Three hybrid orbitals are located symmetrically in space at an angle of 120 0 to each other and directed to the vertices of a regular triangle. Three hybrid orbitals of the boron atom form with one-electron s-orbitals of three hydrogen atoms, three chemical bonds in the BH 3 molecule, which has the shape of a regular triangle (Fig. 4).
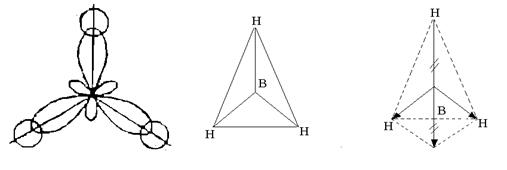
Rice. 4. The structure of the BH 3 molecule
Despite the fact that the B – H bonds are polar (EO B = 2.0; EO H = 2.1), the molecule is non-polar, because has a symmetrical structure. The resulting vector sum of the electric moments of the dipoles of the three B – H bonds is zero (see Fig. 4).
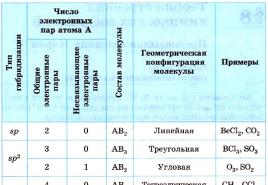
Hybridization of atomic orbitals determines the spatial configuration of molecules and ions (Table 2).
table 2
Spatial structures of some molecules and ions
6.2.5. Explanation of the formation and properties of diatomic molecules of type B 2 by the method of molecular orbitals (MO)
According to the MO method, there are no atomic orbitals (AO) in a molecule, but there are bonding and antibonding MOs obtained by linear combination (mixing) of AOs. When combining n AO is formed n MO, of which the number of bonding agents is equal to the number of AO energy. The energy of binding MOs is less than the energy of AO, and the energy of antibonding MOs is greater than the energy of AO. The procedure for placing electrons on the MO is the same as in the case of AO; first of all, low-energy MOs (minimum energy principle) are filled in accordance with Pauli's principle and Gund's rule.
The simplest case of MO formation occurs when the AO of two atoms of the same element (B2) is combined. With a combination of two s-s AO two MOs are formed, called σ (sigma) orbitals. One of them is binding (), the other is loosening (). Two s-MOs are also obtained by a combination of two p x-p x AO (s). A combination of the two r y-r y and two p z -p z AO is formed by two p sv -MO (and) and two p times -MO (and).
The formation of MOs from atomic ones and the change in their energy can be represented in the form of an energy diagram, where the values of the energy of the orbitals are plotted along the vertical (Fig. 5).
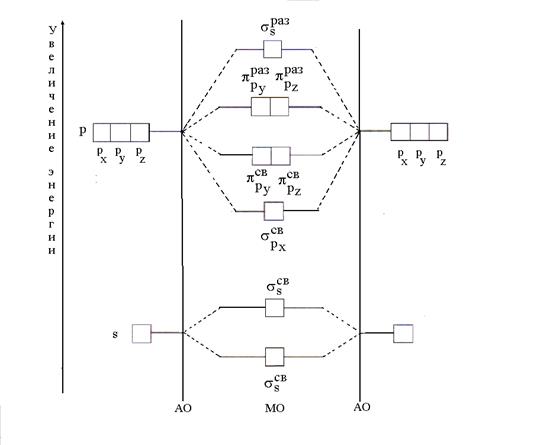
Rice. 5. Energy scheme of MO formation with the combination of AO of two atoms of the same element (B2)
The MO method allows you to determine the magnetism of a molecule. So, the presence of unpaired electrons in a molecule determines its paramagnetism, and the absence of such - diamagnetism. By the MO method, you can find the frequency of communication. The multiplicity w is equal to the half difference of the number of electrons on the bonding n sv and loosening n times MO:
If the number of electrons on the bonding and antibonding MOs is equal, then the bond multiplicity is zero. Consequently, these electrons have no effect on the formation of a chemical bond.
The MO method can also explain the stability of the molecule. The stability of a molecule is associated with the energy balance of all binding and antibonding electrons. Roughly, we can assume that one antibonding electron nullifies the effect of one bonding electron. Thus, the more electrons on bonding MOs and fewer electrons on antibonding MOs, the more stable the molecule.
Example 11. Explain by the MO method the formation of the O 2 molecule. Determine the multiplicity of the connection and its magnetism.
Solution. The O 2 molecule consists of an atom of the same element - oxygen. Its electronic formula is as follows: 8 O 1 s 2 2s 2 2p 4 . Valence electrons of the oxygen atom 2 s 2 2p 4 take part in the formation of a chemical bond, i.e. MOs are obtained by linear combination 2 s- and 2 R- AO of two oxygen atoms.
The distribution of twelve valence electrons in the oxygen molecule occurs in accordance with the principle of minimum energy. First, MOs are filled with two electrons each with antiparallel spins (Pauli's principle). Then six electrons will be placed on three bonding MO (;;), two electrons each with antiparallel spins. The remaining two electrons will be occupied by antibonding MO (s), one electron each with parallel spins according to Gund's rule. After placing the electrons on the MO, the energy diagram of the oxygen molecule will have the following form (Fig. 6).
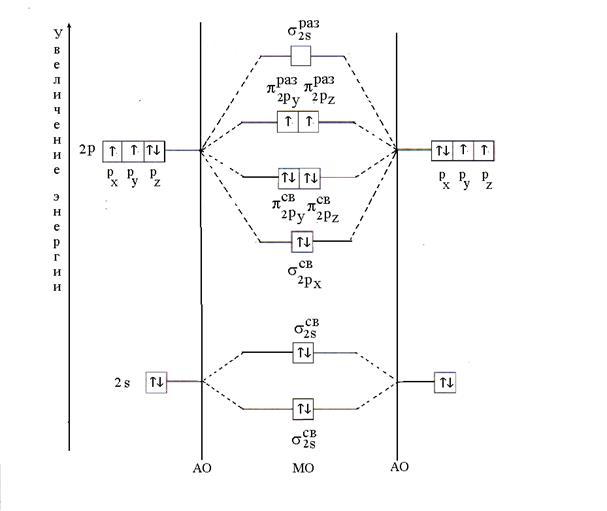
Rice. 6. Energy diagram of the O 2 molecule
The multiplicity of the bond in the oxygen molecule, calculated by the formula (2), is equal to two. The presence of two unpaired electrons determines the paragnetism of the molecule.
CONTROL TASKS
101. In the molecule of ethine (acetylene) C 2 H 2 there is a triple bond between the carbon atoms: one s and two p bonds. The formation of two p bonds occurs due to overlapping r y-r y and two p z -p z atomic orbitals. How does s bond arise? Determine the type of hybridization in the formation of the s bond. Draw the structure of the ethine molecule and calculate the binding energy using the data in Table. 3.
Answer: Δ H= - 245 kJ / mol.
102. Using the energy diagram obtained by the MO method, explain why the molecule is unstable, but the ion is stable. Calculate the frequency of the connection in both cases. How and why does the chemical bond energy change on going to.
103. Explain the formation of chemical bonds in the H2O molecule according to the BC method. Draw the spatial configuration of the molecule. Calculate the length of the Н – О bond using the data in Table 2. Answer: 0.97 · 10 -10 m.
104. Explain the structure of the CH 4 molecule according to the BC method. Determine the type of hybridization in the formation of chemical bonds. Using the data in Table 4, prove that the bonds in the molecule are covalently polar. Why is the electric moment of the dipole of the molecule zero? ... Which atom acts as a donor and which one acts as an acceptor?
108. Explain the structure of the СCl 4 molecule according to the BC method. Determine the type of hybridization in the formation of chemical bonds. Calculate the length of the C – Cl bond in this molecule using the data in Table. 2. Answer: 1.765 · 10 -10 m.
109. Explain the structure of the CO 2 molecule according to the BC method. Draw the spatial configuration of the molecule. Determine the type of hybridization in the formation of chemical bonds. Using the data in the table. 4, prove that the bonds are covalent polar. Why is the CO 2 molecule non-polar?
110. The BF 3 molecule has a planar triangular configuration, while the NF 3 molecule has a three-dimensional (pyramidal) configuration. Using the VS method, explain what is the reason for the difference in the structure of these molecules? Which molecule is non-polar and why? Confirm the result by the values of the electric moments of the dipoles of the molecules (see Table 5).
111. The CH 4 molecule and the ion have the same spatial configuration. How can this be explained in terms of hybridization of the atomic orbitals of the central atom? Why is the electric moment of the dipole of the CH 4 molecule equal to zero?
112. Using the method of valence bonds, explain the structure of the ethane molecule C 2 H 6 (H 3 C – CH 3). What is the type of chemical bond hybridization? Calculate the energy of the C – C bond if the standard enthalpy of formation of C 2 H 6 from gaseous carbon and hydrogen is - 2798.8 kJ / mol, and the energy of the C – H bond is –411.3 kJ / mol.
Answer: Δ H= - 331.0 kJ / mol.
113. Using the BC method, explain why the Н 2 О and Н 2 S molecules have an angular structure, while the bond angle<(H–O–H) = 104 0 30¢ больше валентного угла <(H–S–H) = 92 0 . На основании данных табл.5 вычислите длины диполей молекул и покажите направления электрических моментов диполей связей в них.
Answer: 3.83 · 10 –11 and 1.55 · 10 –11 m.
114. Using the BC method, explain the molecular structure of PCl 3 and AlCl 3. What is the difference in geometric configurations of molecules? Why is PCl 3 molecule polar, and AlCl 3 has zero electric dipole moment?
115. Using the BC method, explain the structure of the CS 2 molecule. Determine the type of hybridization. What is the geometric configuration of the molecule? Why is the electric moment of the dipole zero?
; ... Calculate the frequency of communication and determine which one will be the most stable?120. Using the MO method, build energy diagrams for; ; ... Calculate the multiplicity of bonds and their magnetic properties.