Chemical equilibrium: chemical equilibrium constant and ways of expressing it
In 1885, the French physicist and chemist Le Chatelier was derived, and in 1887 the German physicist Brown substantiated the law of chemical equilibrium and the constant of chemical equilibrium, and also studied their dependence on the influence of various external factors.
The essence of chemical equilibrium
Equilibrium is a state that means things are always in motion. Products are decomposed into reagents and reagents are combined into products. Things move, but the concentration remains the same. The reaction is written with a double arrow instead of an equal sign to indicate that it is reversible.

Classic patterns
Even in the last century, chemists discovered certain patterns that provide for the likelihood of a change in the direction of the reaction in the same container. Knowledge of how chemical reactions take place is incredibly important, both for laboratory research and industrial production. At the same time, the ability to control all these phenomena is of great importance. It is natural for a person to interfere in many natural processes, especially reversible ones, in order to then use them for his own good. Knowledge of chemical reactions will be more useful if you have perfect control over them.
The law of mass action in chemistry is used by chemists to correctly calculate the rates of reactions. It gives a clear idea that none will be followed through in the event that it takes place in a closed system. The molecules of the resulting substances are in constant and disorderly movement, and a reverse reaction may soon occur, in which the molecules of the starting material will be reduced.
In industry, open systems are most often used. Vessels, apparatus and other containers where chemical reactions take place remain unlocked. This is necessary so that during these processes it is possible to extract the desired product and get rid of useless reaction products. For example, coal is burned in open furnaces, cement is produced in open-type furnaces, blast furnaces operate with a constant supply of air, and ammonia is synthesized with the continuous removal of ammonia itself.

Reversible and irreversible chemical reactions
Based on the name, it is possible to give appropriate definitions: reactions are considered irreversible if they are carried through to the end, do not change their direction and proceed along a given trajectory, regardless of pressure drops and temperature fluctuations. Their distinctive feature is that some products can leave the reaction sphere. Thus, for example, it is possible to obtain gas (CaCO 3 = CaO + CO 2), precipitate (Cu (NO 3) 2 + H 2 S = CuS + 2HNO 3) or others will also be considered irreversible if a large amount is released during the process thermal energy, for example: 4P + 5O 2 = 2P 2 O 5 + Q.
Almost all reactions that occur in nature are reversible. Regardless of external conditions such as pressure and temperature, almost all processes can proceed simultaneously in different directions. According to the law of mass action in chemistry, the amount of absorbed heat will be equal to the amount released, which means that if one reaction was exothermic, then the second (reverse) will be endothermic.
Chemical equilibrium: chemical equilibrium constant
Reactions are the "verbs" of chemistry - the activity that chemists study. Many reactions go to their completion and then stop, which means that the reagents are completely converted into products, without being able to return to their original state. In some cases, the reaction is indeed irreversible, for example, when combustion changes both physical and chemical. However, there are many other circumstances in which it is not only possible, but also continuous, since the products of the first reaction become reagents in the second.
A dynamic state in which the concentrations of reactants and products remain constant is called equilibrium. It is possible to predict the behavior of substances using certain laws that are applied in industries seeking to reduce the production costs of specific chemicals. To understand the processes that preserve or potentially threaten human health, the concept of chemical equilibrium is also useful. Chemical equilibrium constant is the value of the reaction factor, which depends on ionic strength and temperature, and does not depend on the concentrations of reagents and products in solution.

Calculating the equilibrium constant
This value is dimensionless, that is, it does not have a certain number of units. Although the calculation is usually written for two reactants and two products, it works for any number of reaction participants. The calculation and interpretation of the equilibrium constant depends on whether the chemical reaction is associated with homogeneous or heterogeneous equilibrium. This means that all reacting components can be pure liquids or gases. For reactions that reach heterogeneous equilibrium, there is usually not one phase, but at least two. For example, liquids and gases or liquids.

Equilibrium constant value
For any given temperature, there is only one value for the equilibrium constant, which changes only if the temperature at which the reaction occurs changes in one direction or another. Some predictions about a chemical reaction can be made based on whether the equilibrium constant is large or small. If the value is very large, then the equilibrium favors the reaction to the right and more products are obtained than there were reagents. The reaction in this case can be called "complete" or "quantitative".
If the value of the equilibrium constant is small, then it favors the reaction to the left, where the amount of reactants was greater than the resulting products. If this value tends to zero, we can assume that the reaction does not occur. If the values of the equilibrium constant for the forward and reverse reactions are almost the same, then the amount of reagents and products will also be almost the same. This type of reaction is considered reversible.
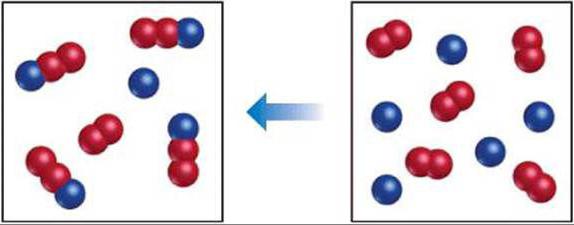
Consider a specific reversible reaction
Let's take two chemical elements such as iodine and hydrogen, which, when mixed, give a new substance - hydrogen iodide.
For v 1 we will take the rate of the forward reaction, for v 2 - the rate of the reverse reaction, k - the equilibrium constant. Using the law of mass action, we get the following expression:
v 1 = k 1 * c (H 2) * c (I 2),
v 2 = k 2 * c 2 (HI).
When iodine (I 2) and hydrogen (H 2) molecules are mixed, their interaction begins. At the initial stage, the concentration of these elements is maximum, but by the end of the reaction, the concentration of the new compound, hydrogen iodide (HI), will be maximum. Accordingly, the reaction rates will also be different. At the very beginning, they will be maximum. Over time, a moment comes when these values are equal, and it is a state called chemical equilibrium.
The expression for the chemical equilibrium constant is usually denoted using square brackets:,,. Since in a state of equilibrium the speeds are equal, then:
k 1 = k 2 2,
so we get the equation for the constant of chemical equilibrium:
k 1 / k 2 = 2 / = K.

Le Chatelier-Brown principle
There is the following pattern: if a certain effect is made on a system that is in equilibrium (to change the conditions of chemical equilibrium by changing temperature or pressure, for example), then the balance will shift to partially counteract the effect of the change. In addition to chemistry, this principle also applies in several different forms to the fields of pharmacology and economics.

Chemical equilibrium constant and ways of expressing it
Equilibrium expression can be expressed in terms of the concentration of products and reagents. Only chemicals in the aqueous and gaseous phases are included in the equilibrium formula, since the concentrations of liquids and solids do not change. What factors affect chemical equilibrium? If a pure liquid or solid is involved in it, it is considered that it has K = 1, and, accordingly, ceases to be taken into account, with the exception of highly concentrated solutions. For example, pure water has an activity of 1.
Another example is solid carbon, which can be formed by the reaction of two molecules of carbon monoxide to form carbon dioxide and carbon. Factors that can affect equilibrium include the addition of a reagent or product (changes in concentration affect balance). The addition of a reagent can lead to equilibrium on the right in the chemical equation where more product forms appear. The addition of product can lead to equilibrium on the left as more forms of reactants become.

Equilibrium occurs when a reaction in both directions has a constant ratio of products and reactants. In general, chemical equilibrium is static, since the quantitative ratio of products and reagents is constant. However, a closer look reveals that balance is actually a very dynamic process, as the reaction moves in both directions at an equal pace.
Dynamic equilibrium is an example of a steady state function. For a system in a steady state, the currently observed behavior continues into the future. Therefore, as soon as the reaction reaches equilibrium, the concentration ratio of the product and reagent will remain the same, although the reaction continues.

How easy is it to tell about the difficult?
Concepts such as chemical equilibrium and chemical equilibrium constant are difficult to understand. Let's take a real life example. Have you ever got stuck on a bridge between two cities and noticed that the traffic in the other direction is smooth and measured, while you are hopelessly stuck in traffic? This is not good.
What if the cars moved steadily and at the same speed on both sides? Would the number of cars in both cities remain constant? When the speed of entry and exit to both cities is the same, and the number of cars in each city is stable over time, this means that the whole process is in dynamic equilibrium.