Halogens: physical properties, chemical properties. The use of halogens and their compounds
The halogens on the periodic table are located to the left of the noble gases. These five toxic non-metallic elements are in group 7 of the periodic table. These include fluorine, chlorine, bromine, iodine, and astatine. Although astatine is radioactive and has only short-lived isotopes, it behaves like iodine and is often referred to as a halogen. Since halogen elements have seven valence electrons, they only need one extra electron to form a full octet. This characteristic makes them more active than other groups of non-metals.
general characteristics
Halogens form diatomic molecules (of the form X 2, where X denotes a halogen atom) - a stable form of the existence of halogens in the form of free elements. The bonds of these diatomic molecules are non-polar, covalent, and single. allow them to easily combine with most elements, so they never occur unbound in nature. Fluorine is the most active halogen, while astatine is the least.
All halogens form Group I salts with similar properties. In these compounds, the halogens are present as halogen anions with a charge of -1 (eg, Cl -, Br -). The ending -id indicates the presence of halide anions; for example Cl - called "chloride".
In addition, the chemical properties of halogens allow them to act as oxidizing agents - to oxidize metals. Most of the chemical reactions in which halogens are involved are redox in an aqueous solution. Halogens form single bonds with carbon or nitrogen, where their oxidation state (CO) is -1. When a halogen atom is replaced by a covalently bonded hydrogen atom in an organic compound, the halo prefix can be used in a general sense, or the prefixes fluoro-, chloro-, bromo-, iodo- - for specific halogens. Halogen elements can cross-link to form diatomic molecules with polar covalent single bonds.
Chlorine (Cl 2) was the first halogen discovered in 1774, followed by iodine (I 2), bromine (Br 2), fluorine (F 2) and astatine (At, the last discovered in 1940). The name "halogen" comes from the Greek roots hal- ("salt") and -gen ("to form"). Together, these words mean "salt-forming", emphasizing the fact that halogens react with metals to form salts. Halite is the name for rock salt, a natural mineral composed of sodium chloride (NaCl). And finally, halogens are used in everyday life - fluoride is contained in toothpaste, chlorine disinfects drinking water, and iodine promotes the production of thyroid hormones.
Chemical elements
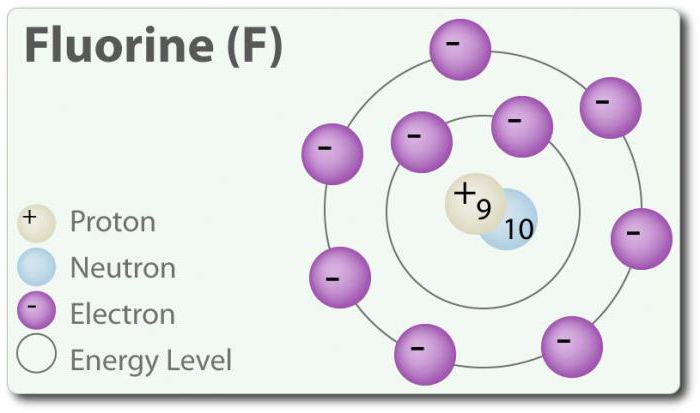
Fluorine is an element with atomic number 9, denoted by the symbol F. Elemental fluorine was first discovered in 1886 by separating it from hydrofluoric acid. In its free state, fluorine exists as a diatomic molecule (F 2) and is the most abundant halogen in the earth's crust. Fluorine is the most electronegative element on the periodic table. It is a pale yellow gas at room temperature. Fluorine also has a relatively small atomic radius. Its CO is -1, except for the elemental diatomic state, in which its oxidation state is zero. Fluorine is extremely reactive and interacts directly with all elements except helium (He), neon (Ne) and argon (Ar). In H 2 O solution, hydrofluoric acid (HF) is a weak acid. Although fluorine is highly electronegative, its electronegativity does not determine acidity; HF is a weak acid due to the fact that the fluorine ion is basic (pH> 7). In addition, fluorine produces very powerful oxidizing agents. For example, fluorine can react with the inert gas xenon to form a strong oxidizing agent, xenon difluoride (XeF 2). Fluoride has many uses.
Chlorine is an element with atomic number 17 and chemical symbol Cl. Discovered in 1774 by isolating it from hydrochloric acid. In its elementary state, it forms a diatomic molecule Cl 2. Chlorine has several COs: -1, +1, 3, 5 and 7. At room temperature, it is a light green gas. Since the bond that forms between two chlorine atoms is weak, the Cl 2 molecule has a very high ability to form compounds. Chlorine reacts with metals to form salts called chlorides. Chlorine ions are the most common ions found in seawater. Chlorine also has two isotopes: 35 Cl and 37 Cl. Sodium chloride is the most abundant of all chlorides.
Bromine is a chemical element with atomic number 35 and symbol Br. It was first discovered in 1826. In its elemental form, bromine is a diatomic molecule of Br 2. It is a reddish brown liquid at room temperature. Its CO is -1, +1, 3, 4 and 5. Bromine is more active than iodine, but less active than chlorine. In addition, bromine has two isotopes: 79 Br and 81 Br. Bromine is found in bromide dissolved in seawater. In recent years, the production of bromide in the world has increased significantly due to its availability and long life span. Like other halogens, bromine is an oxidizing agent and highly toxic.
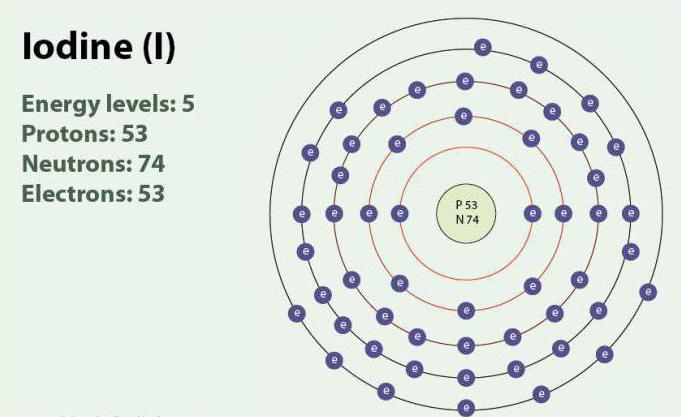
Iodine is a chemical element with atomic number 53 and symbol I. Iodine has oxidation states: -1, +1, +5 and +7. It exists as a diatomic molecule, I 2. At room temperature, it is a purple solid. Iodine has one stable isotope - 127 I. It was first discovered in 1811 with the help of algae and sulfuric acid. Currently, iodine ions can be released in seawater. Despite the fact that iodine is not very soluble in water, its solubility can increase with the use of individual iodides. Iodine plays an important role in the body by participating in the production of thyroid hormones.

Astatine is a radioactive element with atomic number 85 and the symbol At. Its possible oxidation states are -1, +1, 3, 5 and 7. The only halogen that is not a diatomic molecule. Under normal conditions it is a black metallic solid. Astatine is a very rare element, so little is known about it. In addition, astatine has a very short half-life, no longer than a few hours. Obtained in 1940 as a result of synthesis. Astatine is believed to be similar to iodine. Is different
The table below shows the structure of halogen atoms, the structure of the outer layer of electrons.
This structure of the outer layer of electrons makes the physical and chemical properties of halogens similar. At the same time, when comparing these elements, differences are also observed.
Periodic properties in the halogen group
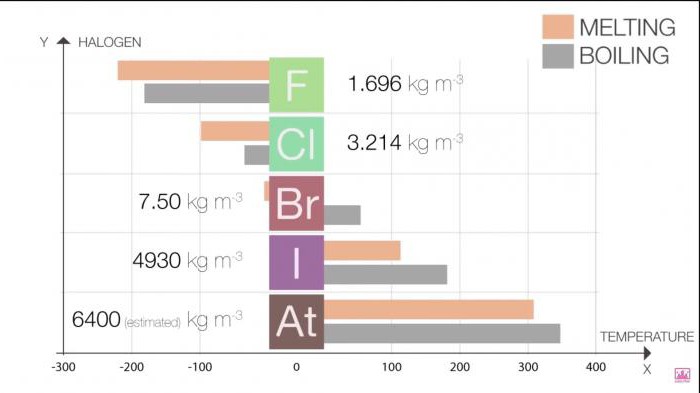
The physical properties of simple substances of halogens change with an increase in the ordinal number of the element. For better assimilation and greater clarity, we offer you several tables.
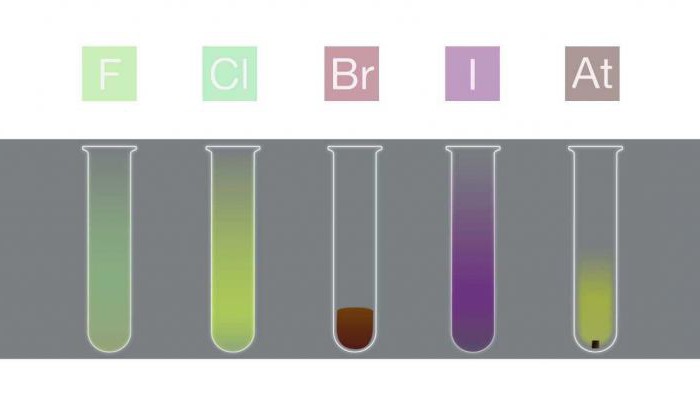
Melting and boiling points in a group increase as the size of the molecule (F Table 1. Halogens. Physical properties: melting and boiling points Halogen Melting point (˚C) Boiling point (˚C) The kernel size increases (F< Cl < Br < I < At), так как увеличивается число протонов и нейтронов. Кроме того, с каждым периодом добавляется всё больше уровней энергии. Это приводит к большей орбитали, и, следовательно, к увеличению радиуса атома. Table 2. Halogens. Physical properties: atomic radii Covalent radius (pm) Ionic (X -) radius (pm) If the outer valence electrons are not near the nucleus, then it does not take much energy to move them away from it. Thus, the energy required to push out the outer electron is not so high in the lower part of the group of elements, since there are more energy levels here. In addition, the high ionization energy causes the element to exhibit non-metallic qualities. Iodine and display astatine exhibit metallic properties because the ionization energy decreases (At< I < Br < Cl < F). Table 3. Halogens. Physical properties: ionization energy The number of valence electrons in an atom increases with increasing energy levels at progressively lower levels. The electrons are progressively farther away from the nucleus; Thus, the nucleus and electrons are not both attracted to each other. An increase in shielding is observed. Therefore, Electronegativity decreases with increasing period (At< I < Br < Cl < F). Table 4. Halogens. Physical properties: electronegativity Since the size of the atom increases with the period, the electron affinity, as a rule, decreases (B< I < Br < F < Cl). Исключение - фтор, сродство которого меньше, чем у хлора. Это можно объяснить меньшим размером фтора по сравнению с хлором. Table 5. Electron affinity of halogens The reactivity of halogens decreases with increasing period (At A halide is formed when a halogen reacts with another, less electronegative element to form a binary compound. Hydrogen reacts with halogens to form HX halides: Hydrogen halides readily dissolve in water to form hydrogen halide (hydrofluoric, hydrochloric, hydrobromic, hydroiodic) acid. The properties of these acids are shown below. Acids are formed by the following reaction: HX (aq) + H 2 O (l) → X - (aq) + H 3 O + (aq). All hydrogen halides form strong acids with the exception of HF. The acidity of hydrohalic acids increases: HF Hydrofluoric acid is capable of engraving glass and some inorganic fluorides for a long time. It may seem counterintuitive that HF is the weakest hydrohalic acid, since fluorine has the highest electronegativity. However, the H-F bond is very strong, making the acid very weak. A strong bond is determined by a short bond length and high dissociation energy. Of all hydrogen halides, HF has the shortest bond length and the highest bond dissociation energy. Halogen oxo acids are acids with hydrogen, oxygen and halogen atoms. Their acidity can be determined by structural analysis. Halogen oxo acids are listed below: In each of these acids, a proton is bonded to an oxygen atom, so a comparison of the proton bond lengths is useless here. The dominant role here is played by electronegativity. The acidic activity increases with an increase in the number of oxygen atoms associated with the central atom. The basic physical properties of halogens can be summarized in the following table. State of matter (at room temperature) Halogen Appearance purple red-brown gaseous pale yellow brown pale green The color of halogens is the result of the absorption of visible light by molecules, which causes the electrons to be excited. Fluorine absorbs violet light and therefore appears light yellow. Iodine, on the other hand, absorbs yellow light and appears purple (yellow and violet are complementary colors). The color of halogens becomes darker with increasing period. In closed containers, liquid bromine and solid iodine are in equilibrium with their vapors, which can be observed as a colored gas. Although the color of astatine is unknown, it is assumed that it should be darker than iodine (i.e. black) in accordance with the observed pattern. Now, if you are asked, "Describe the physical properties of halogens," you will have something to say. The oxidation state is often used instead of the term "halogen valence". Typically, the oxidation state is -1. But if a halogen is bonded to oxygen or another halogen, it can take on other states: CO oxygen-2 has priority. In the case of two different halogen atoms bonded together, the more electronegative atom prevails and takes CO -1. For example, in iodine chloride (ICl), chlorine has CO -1 and iodine +1. Chlorine is more electronegative than iodine, so its CO is -1. In bromic acid (HBrO 4), oxygen has CO -8 (-2 x 4 atoms = -8). Hydrogen has a general oxidation state of +1. The addition of these values gives a CO of -7. Since the final CO of the compound must be zero, the CO of bromine is +7. The third exception to the rule is the oxidation state of halogen in elemental form (X 2), where its CO is zero. Halogen CO in compounds 1, +1, +3, +5, +7 1, +1, +3, +4, +5 1, +1, +3, +5, +7 Electronegativity increases with increasing period. Therefore, fluorine has the highest electronegativity of all elements, as evidenced by its position in the periodic table. Its electronic configuration is 1s 2 2s 2 2p 5. If fluorine gets another electron, the outermost p-orbitals are completely filled and make up a full octet. Since fluorine is highly electronegative, it can easily take an electron away from a neighboring atom. Fluorine in this case is isoelectronic to an inert gas (with eight valence electrons), all of its outer orbitals are filled. In this state, fluorine is much more stable. In nature, halogens are in the state of anions, therefore free halogens are obtained by oxidation by electrolysis or using oxidants. For example, chlorine is produced by hydrolysis of sodium chloride solution. The use of halogens and their compounds is diverse.
Hydrogen + halogens
Halogen oxo acids
Appearance and state of matter
Explanation of appearance
Oxidation state of halogens in compounds
Why is CO of fluorine always -1?
Obtaining and using halogens