9.2 Standard hydrogen electrode. Electrode potential.
It is possible to measure with high accuracy the EMF of a circuit composed of two electrodes. However, one can neither measure nor calculate the absolute potential difference at the metal-solution interface. For practical purposes, it is sufficient to have conditional values characterizing the potentials of various electrodes, referred to the potential of a certain electrode chosen as a standard one.
As conditionally zero capacity selected potential of a standard hydrogen electrode:
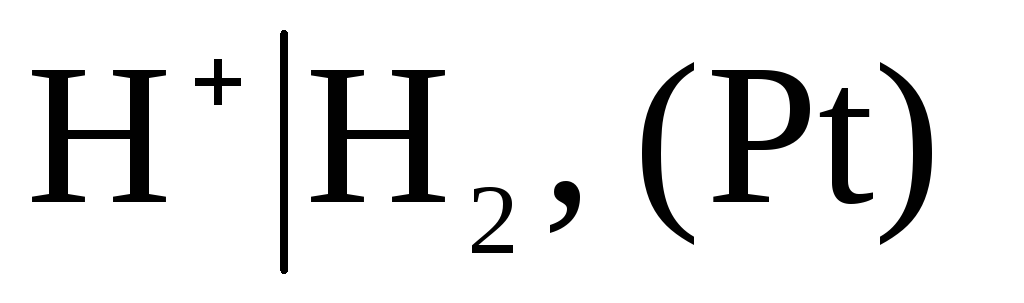 ,
,
in which the pressure of the blown hydrogen is 1 atm, and the activity of hydrogen ions in the solution is equal to unity (Fig. 1). At any temperature 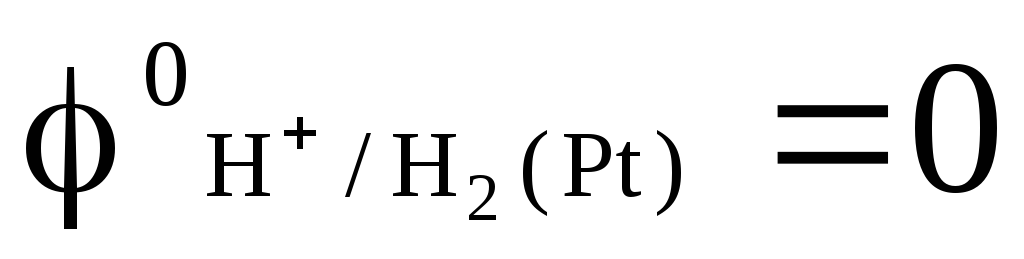 .
.
The electrode reaction for a hydrogen electrode is written as follows:
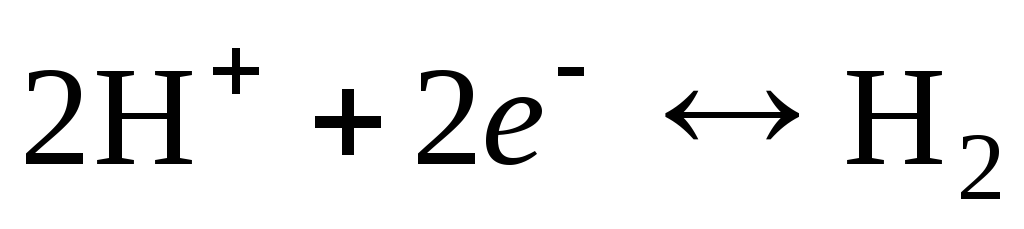 .
.
![]()
Rice. 1. General view of the hydrogen electrode.
For the electrode potential of any electrode, it was decided to take the EMF of a circuit composed of the electrode in question and a standard hydrogen electrode. In this case, the electrode under consideration is located on the right in the circuit, and a standard hydrogen electrode is located on the left.
Example 9.1. Make up a GE to determine the standard electrode potential of Cu 2+ / Cu and Zn 2+ / Zn electrodes.
1). Let's make a galvanic cell from standard hydrogen and copper electrodes:
Total potential-forming reaction:, i.e. the transfer of electrons in the circuit occurs from left to right (from the left electrode to the right). Under standard conditions 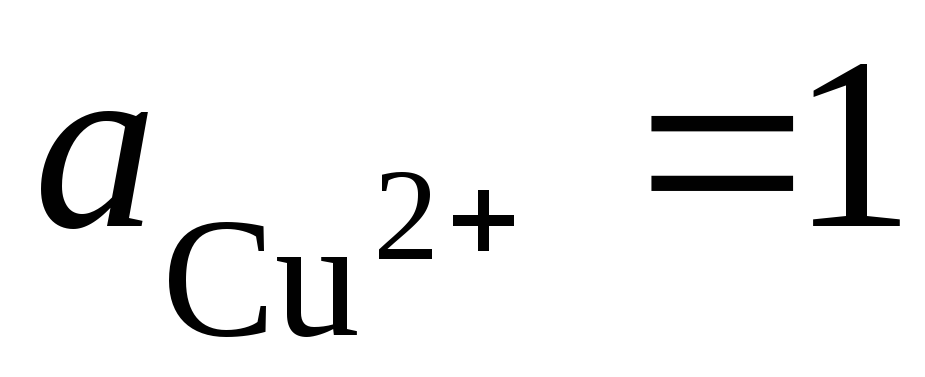 , The EMF of this element will be E = 0.337 V. Therefore,
, The EMF of this element will be E = 0.337 V. Therefore,
![]() .
.
2). In a similar way, let's leave a galvanic cell made of standard hydrogen and zinc electrodes:
Total reaction:, i.e. the transfer of electrons in the circuit occurs from right to left. Under standard conditions 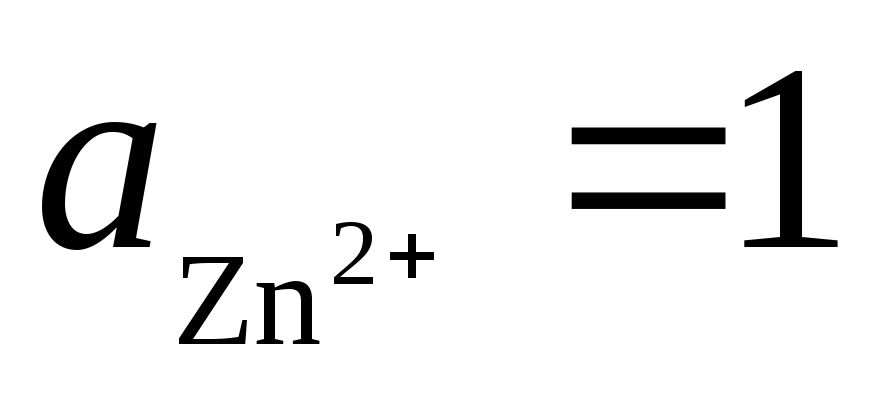
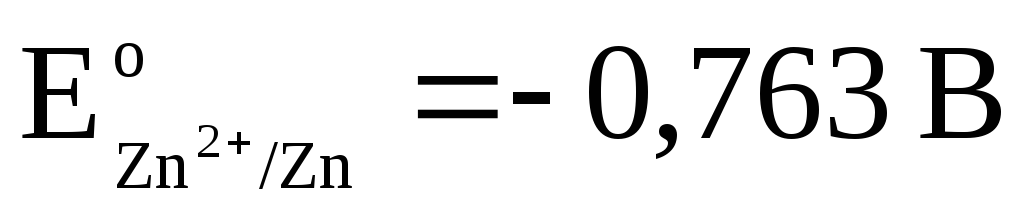 .
.
9.3 Nernst equation for the electromotive force of a galvanic cell.
The electrical characteristic of the electrode is electrode potential, and a galvanic cell (electrochemical circuit) - electromotive force (EMF),
The EMF of a correctly open electrochemical circuit in the absence of a diffusion potential corresponds to the potential difference of the right (positive) and left (negative) electrodes and is always positive.
EMF of a galvanic cell
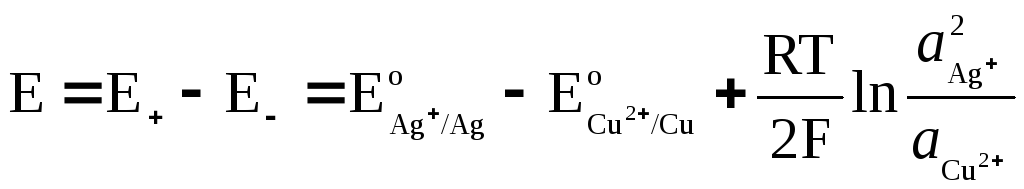
where 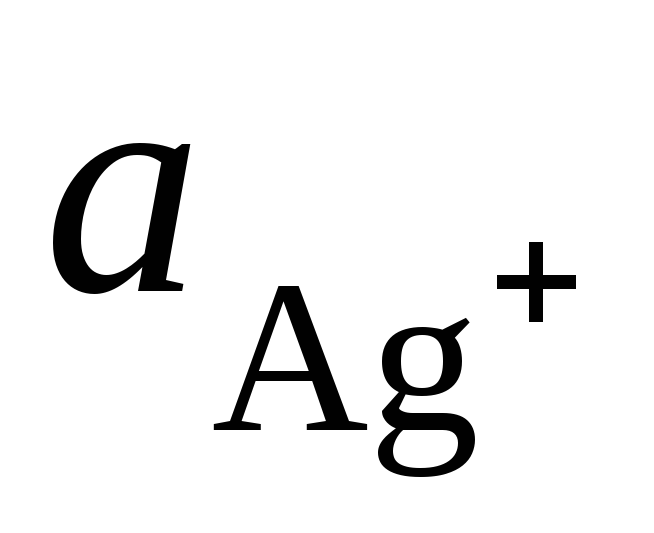 and
and 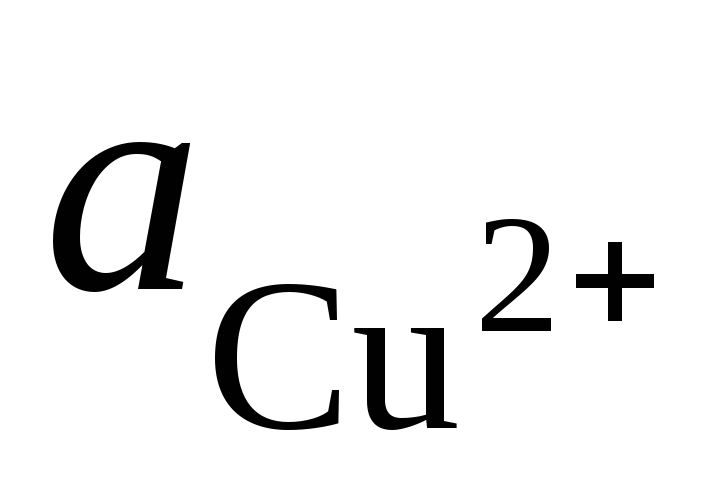 - activity of Ag + and Cu 2+ ions in solutions of their salts.
- activity of Ag + and Cu 2+ ions in solutions of their salts.
We denote
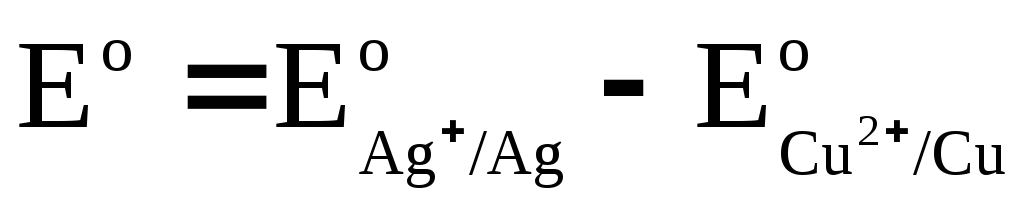 ,
,
where E o - standard EMF of a galvanic cell. Then
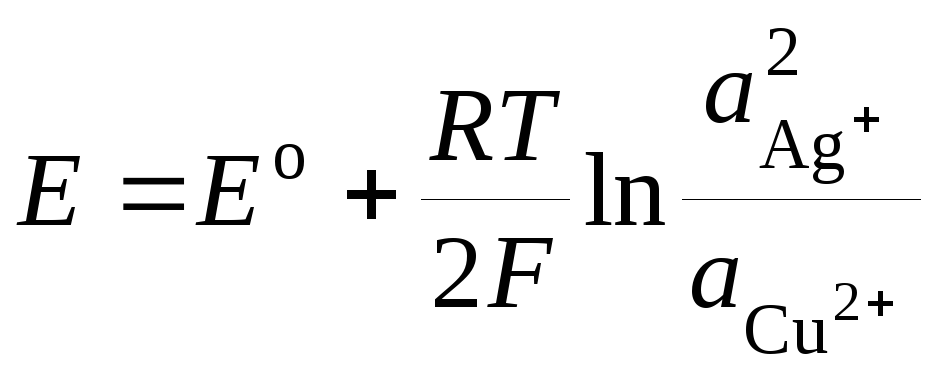 .(3)
.(3)
Equation (3) is called Nernst equations for the EMF of a galvanic cell.
If a reversible chemical reaction takes place in a galvanic cell
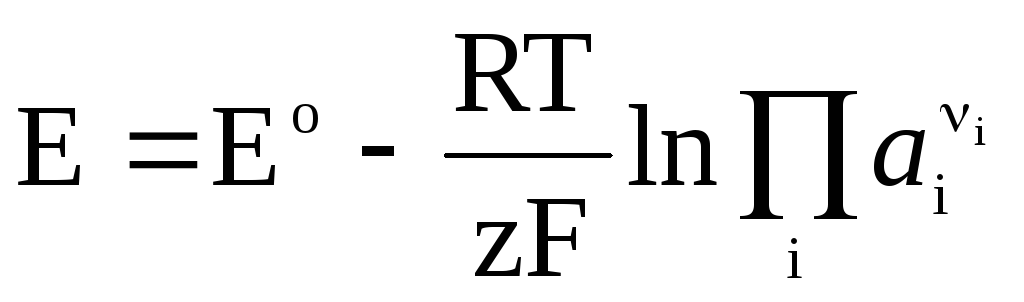 , (4)
, (4)
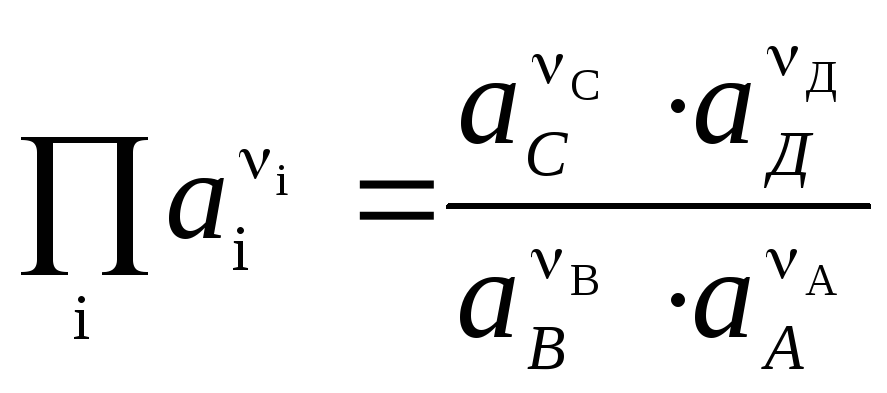
The Nernst equation in the form (4) is applicable to calculate the EMF of any galvanic cell (by the total chemical reaction) and the potential of any electrode (by the electrode reaction). EMF is related to the thermodynamic characteristics of the reaction. It is known that
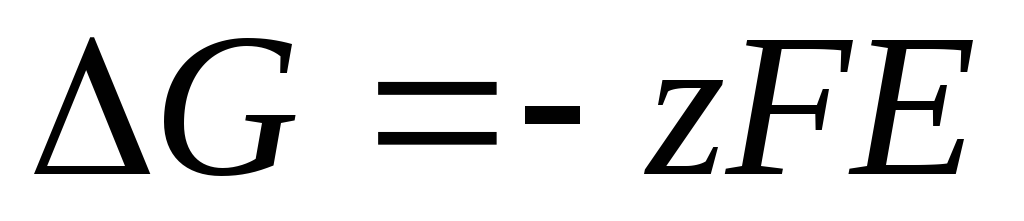 .
.
9.4 Classification of reversible electrodes.
The classification of reversible electrodes is based on the properties of substances involved in potential-determining processes.
1. Electrodes of the first kind.
First class electrodes Are metal electrodes reversible with respect to cations (including amalgam ones) ( 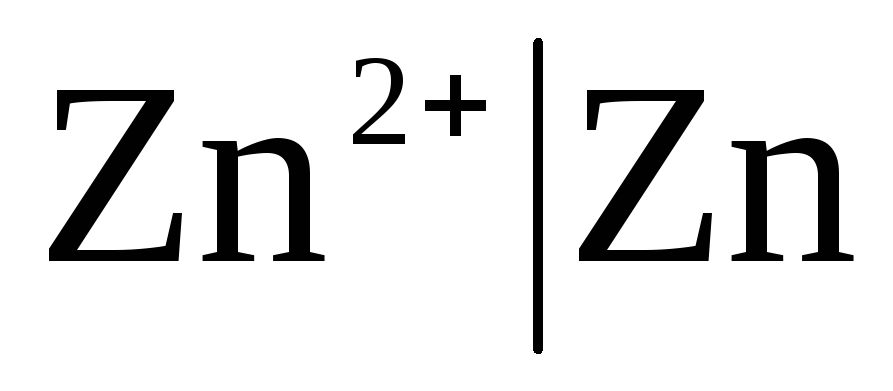 ) and metalloid, electrodes are reversible with respect to anions (
) and metalloid, electrodes are reversible with respect to anions ( 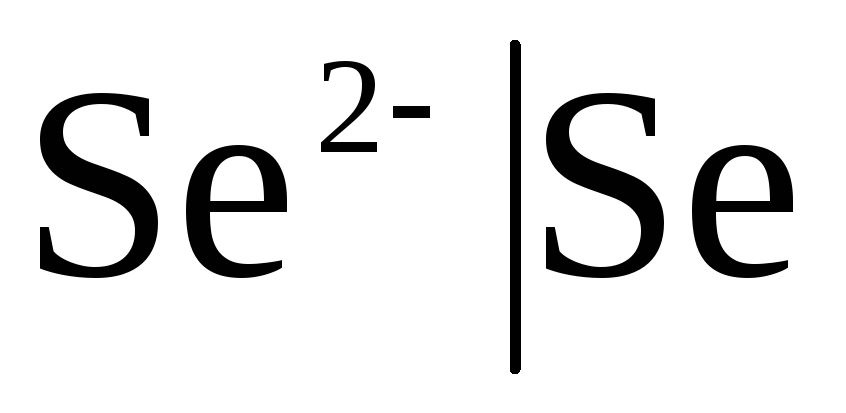 ).
).
If the electrode is reversible with respect to the Zn 2+ / Zn cation, then for it one can write:
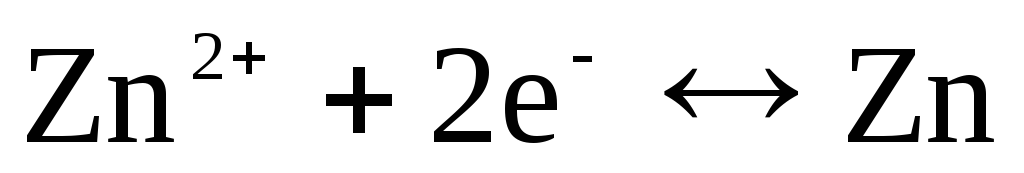 ,
,
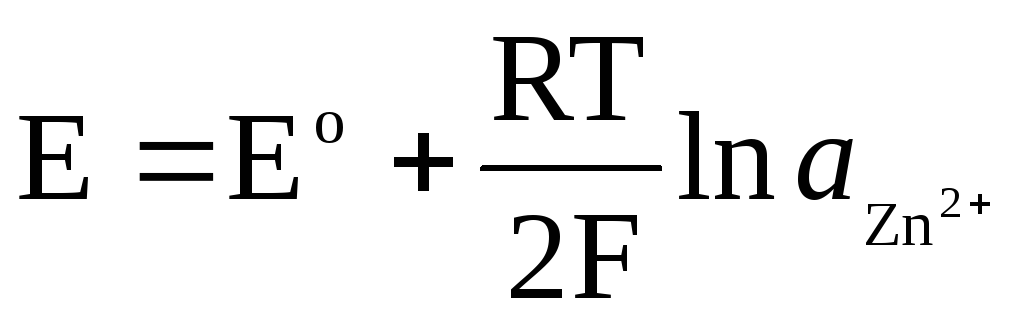
For Zn 2+ / Zn (Hg) amalgam electrode:
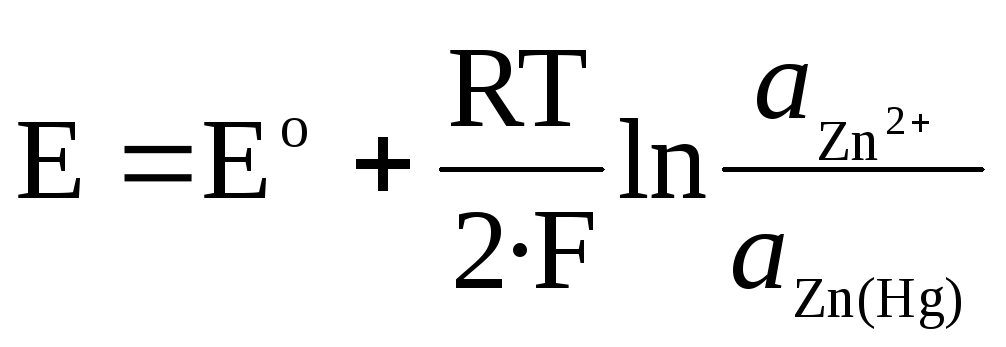 .
.
If the electrode is reversible with respect to the anion, then
![]() ,
,
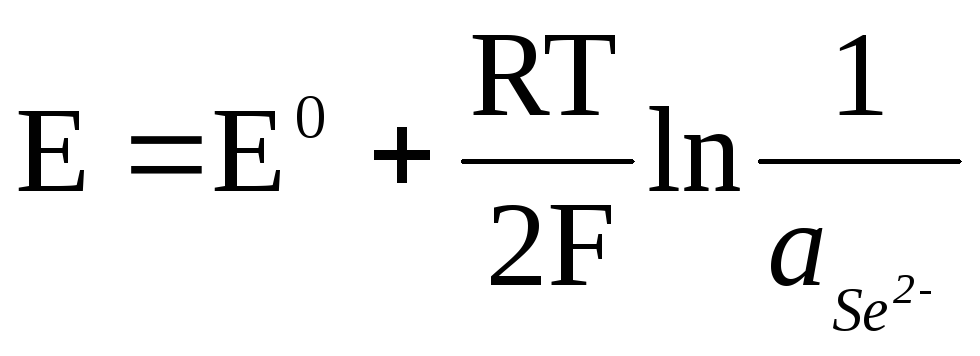 .
.
The electrodes of the first kind also include gas electrodes (hydrogen 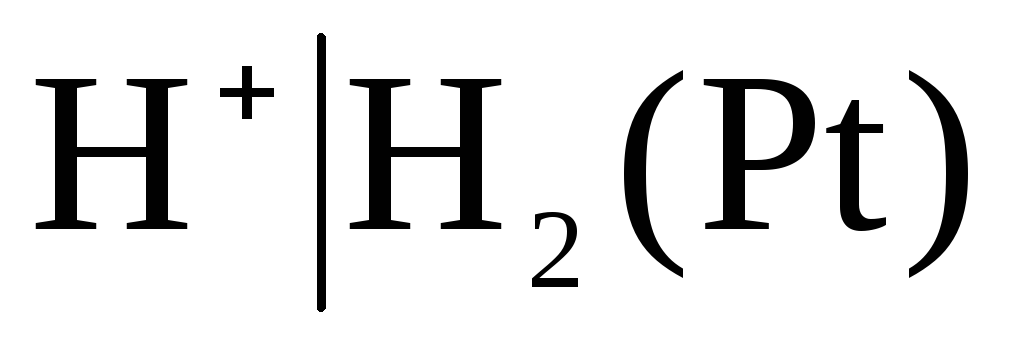 , chlorine
, chlorine 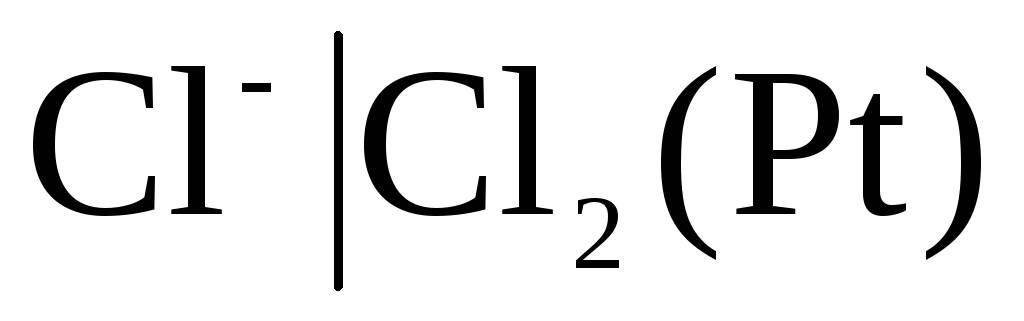 and etc.). They can be reversible with respect to the cation (H +) or anion (Cl -). For example, a hydrogen electrode is reversible with respect to a cation. The equation of the electrode process for it can be written in the form:
and etc.). They can be reversible with respect to the cation (H +) or anion (Cl -). For example, a hydrogen electrode is reversible with respect to a cation. The equation of the electrode process for it can be written in the form:
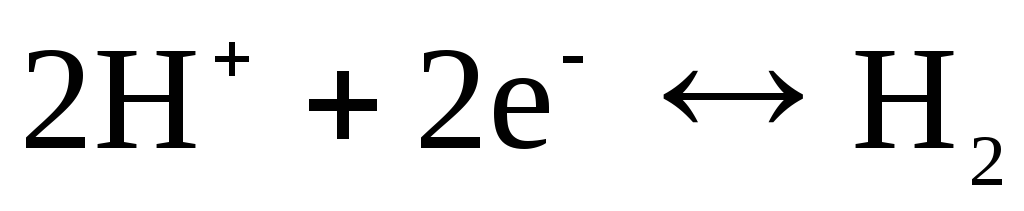 .
.
The potential of the hydrogen electrode is determined by the expression
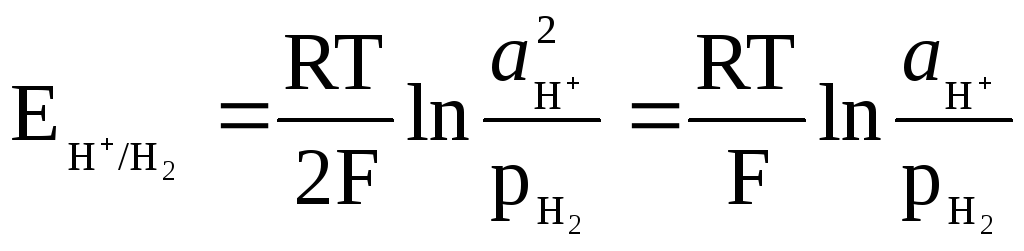 ,
,
and can be related to the pH of the solution.
For chlorine electrode 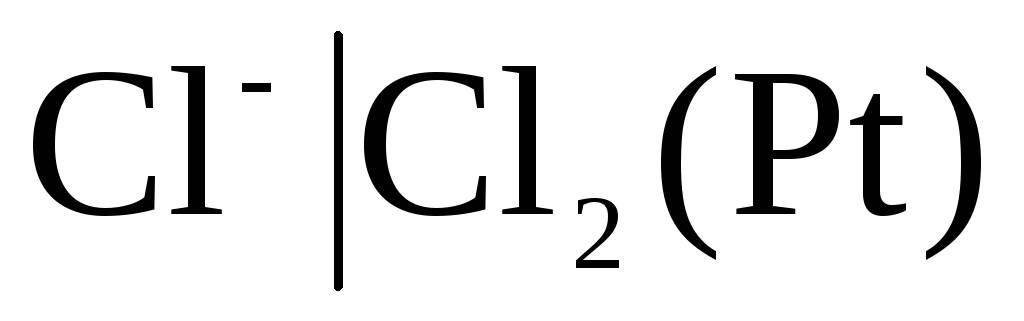 with potential-determining response
with potential-determining response
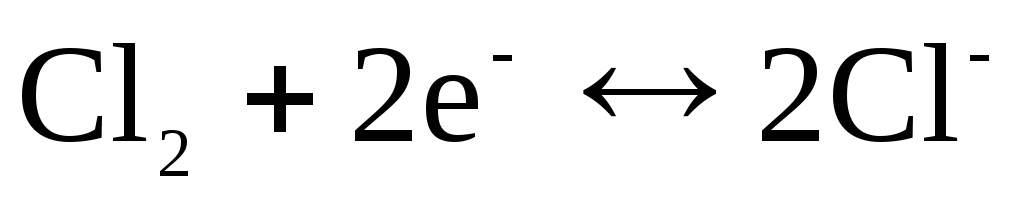 .
.
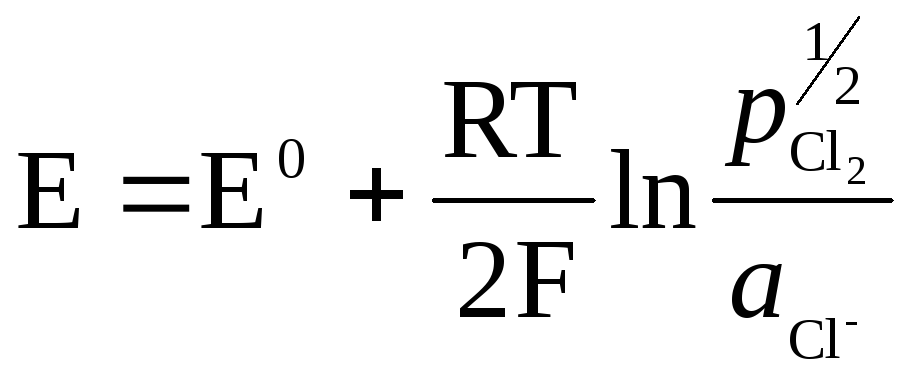
The metal (Pt) in gas electrodes is necessary to create contact between the gas and the solution, must be inert with respect to the substances in the solution, and can catalyze only one potential-determining process.