4. Influence of temperature on the rate of chemical reactions
From qualitative considerations, it is clear that the reaction rate should increase with increasing temperature, because this increases the energy of the colliding particles and increases the likelihood that the collision will result in a chemical transformation. For a quantitative description of temperature effects in chemical kinetics, two basic relations are used - the Van't Hoff rule and the Arrhenius equation.
Van't Hoff's rule lies in the fact that when heated to 10 about C, the rate of most chemical reactions increases 2 to 4 times. Mathematically, this means that the reaction rate depends on temperature in a power-law manner:
![]() , (4.1)
, (4.1)
where is the temperature coefficient of speed (= 24). Van't Hoff's rule is very rough and only applies in a very limited temperature range.
Much more accurate is Arrhenius equation describing the temperature dependence of the rate constant:
![]() , (4.2)
, (4.2)
where R- universal gas constant; A- preexponential factor, which does not depend on temperature, but is determined only by the type of reaction; E A - activation energy, which can be characterized as a certain threshold energy: roughly speaking, if the energy of colliding particles is less E A, then in a collision, the reaction will not occur if the energy exceeds E A, the reaction will happen. The activation energy is independent of temperature.
Graphically addiction k(T) as follows:
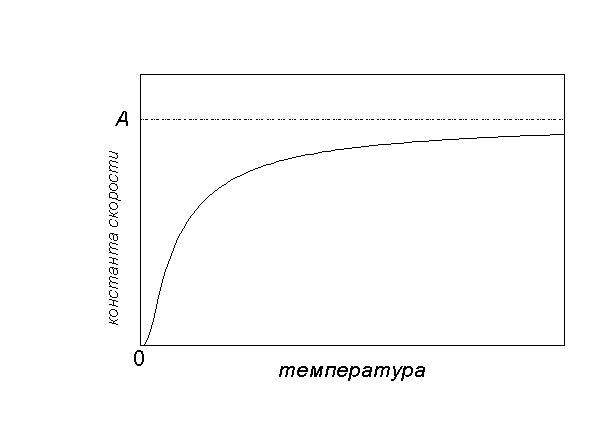
At low temperatures, chemical reactions almost do not occur: k(T) 0. At very high temperatures, the rate constant tends to a limiting value: k(T)A... This corresponds to the fact that all molecules are reactive and each collision leads to a reaction.
The activation energy can be determined by measuring the rate constant at two temperatures. Equation (4.2) implies:
![]() . (4.3)
. (4.3)
More precisely, the activation energy is determined from the values of the rate constant at several temperatures. For this, the Arrhenius equation (4.2) is written in the logarithmic form
![]()
and write the experimental data in coordinates ln k - 1/T... The tangent of the angle of inclination of the resulting straight line is - E A / R.
For some reactions, the preexponential factor is weakly dependent on temperature. In this case, the so-called experimental activation energy:
![]() . (4.4)
. (4.4)
If the preexponential factor is constant, then the experimental activation energy is equal to the Arrhenius activation energy: E op = E A.
Example 4-1. Using the Arrhenius equation, estimate at what temperatures and activation energies the Van't Hoff rule is valid.
Solution. We represent the Van't Hoff rule (4.1) as a power-law dependence of the rate constant:
![]() ,
,
where B- constant value. Let us compare this expression with the Arrhenius equation (4.2), taking the value of ~ e = 2.718:
![]() .
.
Take the natural logarithm of both sides of this approximate equality:
![]() .
.
Differentiating the obtained ratio with respect to temperature, we find the desired relationship between the activation energy and temperature:
If the activation energy and temperature approximately satisfy this ratio, then the Van't Hoff rule can be used to estimate the effect of temperature on the reaction rate.
Example 4-2. The first-order reaction at a temperature of 70 ° C is completed by 40% in 60 minutes. At what temperature is the reaction 80% complete in 120 minutes if the activation energy is 60 kJ / mol?
Solution. For a first-order reaction, the rate constant is expressed in terms of the conversion as follows:
![]() ,
,
where a = x/a- the degree of conversion. We write this equation at two temperatures, taking into account the Arrhenius equation:
where E A= 60 kJ / mol, T 1 = 343 K, t 1 = 60 min, a 1 = 0.4, t 2 = 120 min, a 2 = 0.8. Divide one equation by another and logarithm:
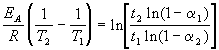
Substituting the above values into this expression, we find T 2 = 333 K = 60 o C.
Example 4-3. The rate of bacterial hydrolysis of fish muscles doubles when going from a temperature of -1.1 o C to a temperature of +2.2 o C. Estimate the activation energy of this reaction.
Solution. A twofold increase in the rate of hydrolysis is due to an increase in the rate constant: k 2 = 2k 1 . The activation energy with respect to the ratio of the rate constants at two temperatures can be determined from equation (4.3) with T 1 = t 1 + 273.15 = 272.05 K, T 2 = t 2 + 273.15 = 275.35 K:
![]() 130800 J / mol = 130.8 kJ / mol.
130800 J / mol = 130.8 kJ / mol.
4-1. Using the Van't Hoff rule, calculate at what temperature the reaction will end in 15 minutes, if at 20 ° C it takes 2 hours. The temperature coefficient of the rate is 3. (answer)
4-2. The half-life of the substance at 323 K is 100 min, and at 353 K it is 15 min. Determine the temperature coefficient of speed. (Answer)
4-3. What should be the activation energy for the reaction rate to increase 3 times with an increase in temperature by 10 0 С a) at 300 K; b) at 1000 K? (answer)
4-4. The first-order reaction has an activation energy of 25 kcal / mol and a preexponential factor of 5. 10 13 sec -1. At what temperature the half-life for this reaction will be: a) 1 min; b) 30 days? (answer)
4-5. In which of the two cases the reaction rate constant increases more times: when heated from 0 o C to 10 o C or when heated from 10 o C to 20 o C? Justify the answer using the Arrhenius equation. (Answer)
4-6. The activation energy of some reaction is 1.5 times higher than the activation energy of another reaction. When heated from T 1 to T 2 the rate constant of the second reaction increased by a once. How many times did the rate constant of the first reaction increase when heated from T 1 to T 2? (Answer)
4-7. The rate constant of a complex reaction is expressed in terms of the rate constants of elementary stages as follows:
Express the activation energy and the preexponential factor of a complex reaction in terms of the corresponding quantities related to the elementary stages. (Answer)
4-8. In an irreversible first order reaction in 20 minutes at 125 ° C, the conversion of the starting material was 60%, and at 145 ° C the same conversion was achieved in 5.5 minutes. Find the rate constants and activation energy of this reaction. (Answer)
4-9. The first order reaction at a temperature of 25 ° C is completed by 30% in 30 minutes. At what temperature will the reaction be completed by 60% in 40 minutes if the activation energy is 30 kJ / mol? (Answer)
4-10. The first order reaction at a temperature of 25 ° C is completed by 70% in 15 minutes. At what temperature will the reaction be completed by 50% in 15 minutes if the activation energy is 50 kJ / mol? (Answer)
4-11. The first order reaction rate constant is 4.02. 10 -4 s -1 at 393 K and 1.98. 10 -3 s -1 at 413 K. Calculate the pre-exponential factor for this reaction. (Answer)
4-12. For the reaction H 2 + I 2 2HI, the rate constant at a temperature of 683 K is 0.0659 L / (mol min), and at a temperature of 716 K - 0.375 L / (mol min). Find the activation energy of this reaction and the rate constant at a temperature of 700 K. (answer)
4-13. For the reaction 2N 2 O 2N 2 + O 2, the rate constant at a temperature of 986 K is 6.72 L / (mol min), and at a temperature of 1165 K - 977.0 L / (mol min). Find the activation energy of this reaction and the rate constant at a temperature of 1053.0 K. (answer)
4-14. Trichloroacetate ion in ionizing solvents containing H + decomposes according to the equation
H + + CCl 3 COO - CO 2 + CHCl 3
The stage that determines the reaction rate is the monomolecular cleavage of the C - C bond in the trichloroacetate ion. The reaction proceeds according to the first order, and the rate constants have the following meanings: k= 3.11. 10 -4 s -1 at 90 о С, k= 7.62. 10 -5 s -1 at 80 o C. Calculate a) activation energy, b) rate constant at 60 o C. (answer)
4-15. For the reaction CH 3 COOC 2 H 5 + NaOH * CH 3 COONa + C 2 H 5 OH, the rate constant at 282.6 K is 2.307 L / (mol min), and at 318.1 K, 21.65 L / (mol min). Find the activation energy of this reaction and the rate constant at a temperature of 343 K. (answer)
4-16. For the reaction C 12 H 22 O 11 + H 2 OC 6 H 12 O 6 + C 6 H 12 O 6, the rate constant at a temperature of 298.2 K is 0.765 L / (mol.min), and at a temperature of 328.2 K - 35.5 L / (mol min). Find the activation energy of this reaction and the rate constant at a temperature of 313.2 K. (answer)
4-17. The substance decomposes in two parallel paths with rate constants k 1 and k 2. What is the difference between the activation energies of these two reactions, if at 10 o C k 1 /k 2 = 10, and at 40 o C k 1 /k 2 = 0.1? (Answer)
4-18. In two reactions of the same order, the difference in activation energies is E 2 - E 1 = 40 kJ / mol. At a temperature of 293 K, the ratio of the rate constants is k 1 /k 2 = 2. At what temperature will the rate constants become equal? (Answer)
4-19. The decomposition of acetone dicarboxylic acid in aqueous solution is a first order reaction. The rate constants of this reaction were measured at different temperatures:
Calculate the activation energy and pre-exponential factor. What is the half-life at 25 ° C?