3.2. Partial pressures in the gas mixture. Dalton's Law
The gas mixture is in a state of equilibrium if the concentrations of the components and its state parameters in the entire volume have the same values. In this case, the temperature of all gases entering the mixture is the same and is equal to the temperature of the mixture T cm.
In an equilibrium state, the molecules of each gas are dispersed evenly throughout the entire volume of the mixture, that is, they have their own specific concentration and, therefore, their own pressure R i, Pa, which is called partial ... It is defined as follows.
The partial pressure is equal to the pressure of a given component, provided that it alone occupies the entire volume intended for the mixture at the mixture temperature T cm .
According to the law of the English chemist and physicist Dalton, formulated in 1801, the pressure of a mixture of ideal gases p cm is equal to the sum of the partial pressures of its components p i :
where n- the number of components.
Expression (2) is also called the law of partial pressures.
3.3. The reduced volume of the gas mixture component. Amag's law
By definition, the reduced volume i th component of the gas mixture V i, m 3, is the volume that this one component could occupy provided that its pressure and temperature are equal to the pressure and temperature of the entire gas mixture.
The law of the French physicist Amag, formulated around 1870, states: the sum of the reduced volumes of all components of the mixture is equal to the volume of the mixtureV cm :
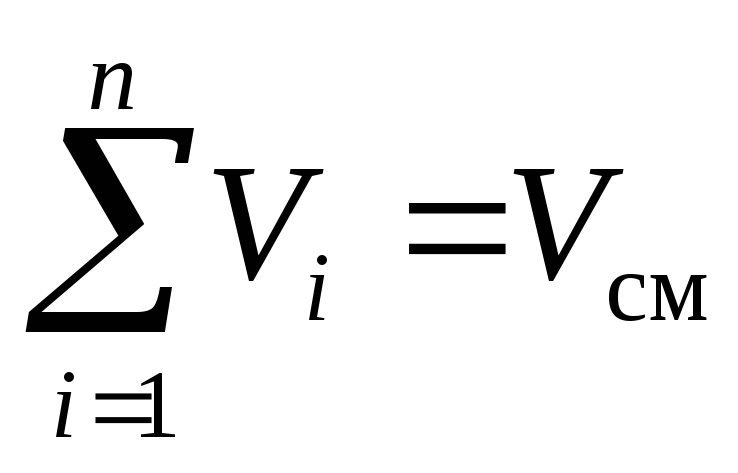 , m 3. (3)
, m 3. (3)
3.4. The chemical composition of the gas mixture
The chemical composition of the gas mixture can be set three different ways.
Consider a gas mixture consisting of n components. The mixture takes up volume V cm, m 3, has a mass M cm, kg, pressure R cm, Pa and temperature T cm, K. Also, the number of moles of the mixture is N cm, mole. Moreover, the mass of one i th component m i, kg, and the number of moles of this component ν i, mole.
It's obvious that:
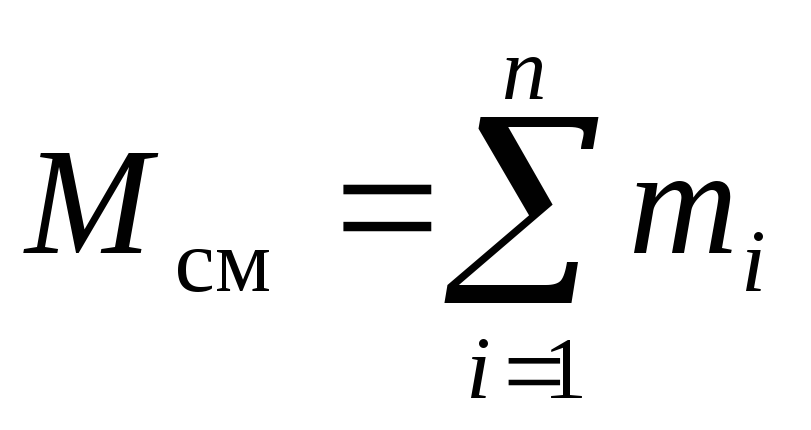 , (4)
, (4)
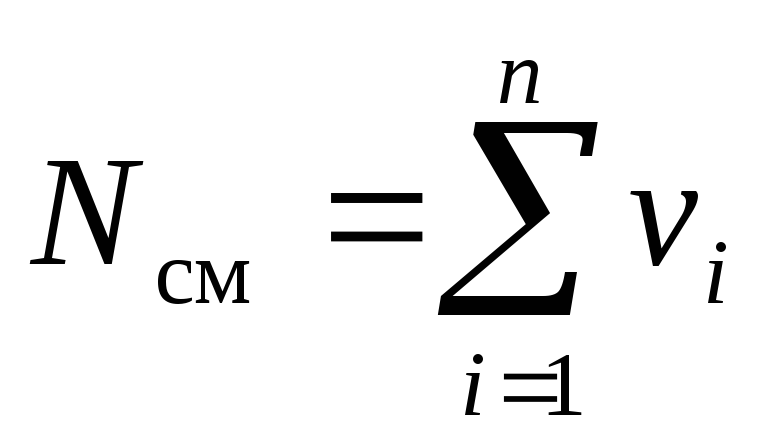 . (5)
. (5)
Using Dalton's law (2) and Amag's law (3) for the mixture under consideration, we can write:
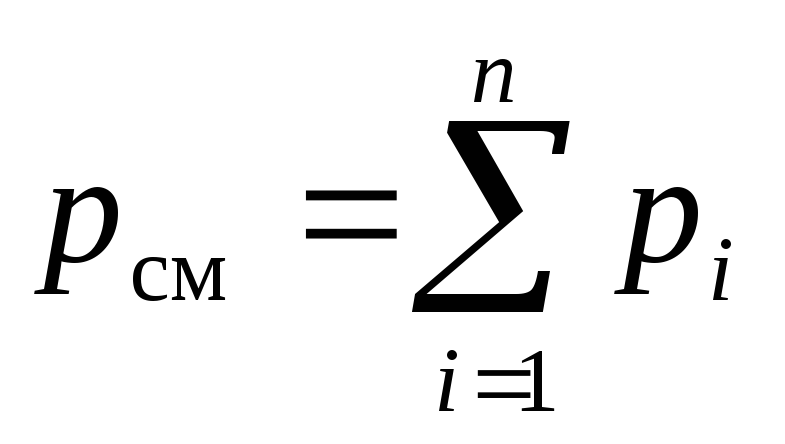 , (6)
, (6)
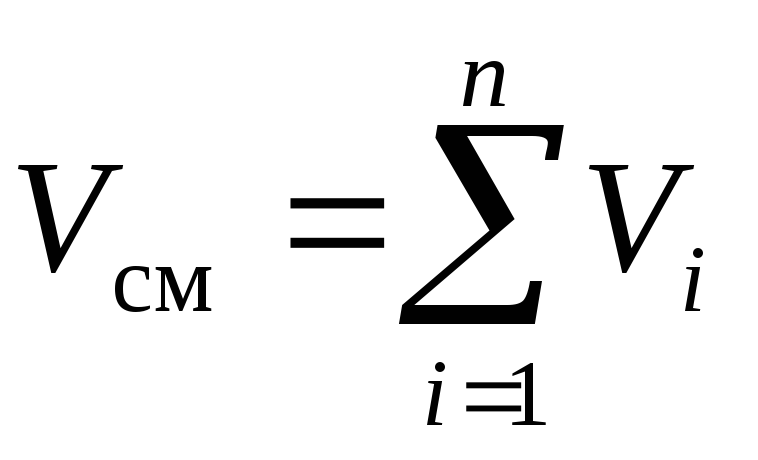 , (7)
, (7)
where R i- partial pressure i th component, Pa; V i- reduced volume i th component, m 3.
The chemical composition of a gas mixture can be unambiguously set either by mass, molar, or volume fractions of its components:
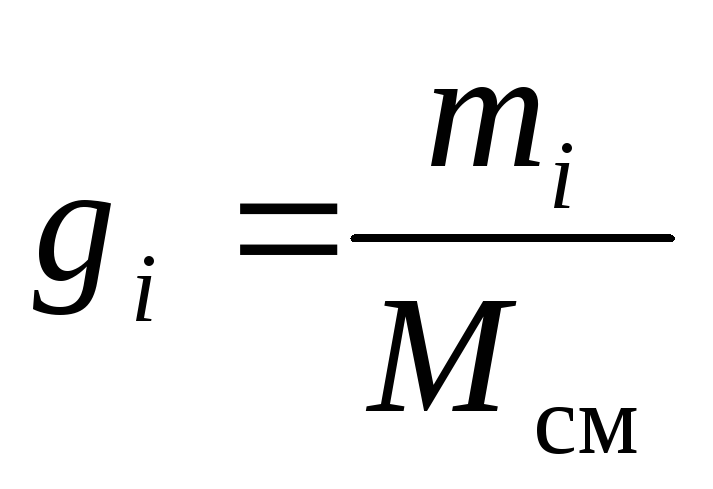 , (8)
, (8)
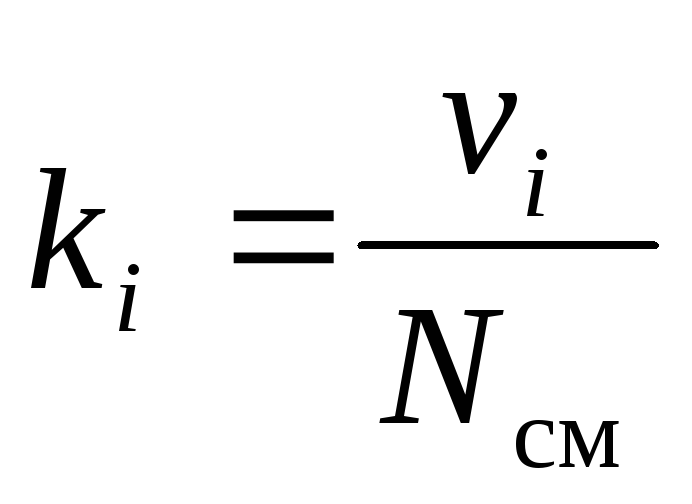 , (9)
, (9)
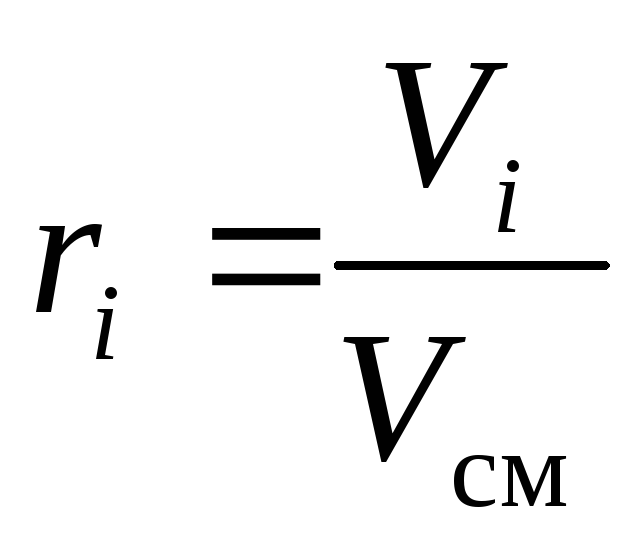 , (10)
, (10)
where g i , k i and r i- mass, molar and volume fraction i-th component of the mixture, respectively (dimensionless quantities).
It's obvious that:
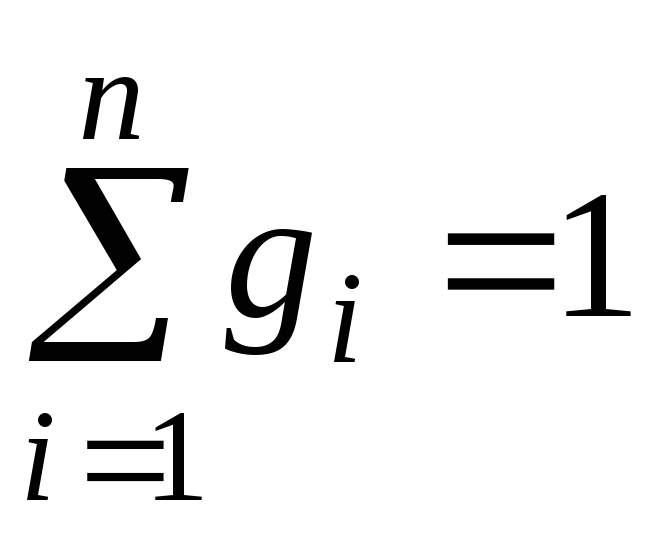 ,
,
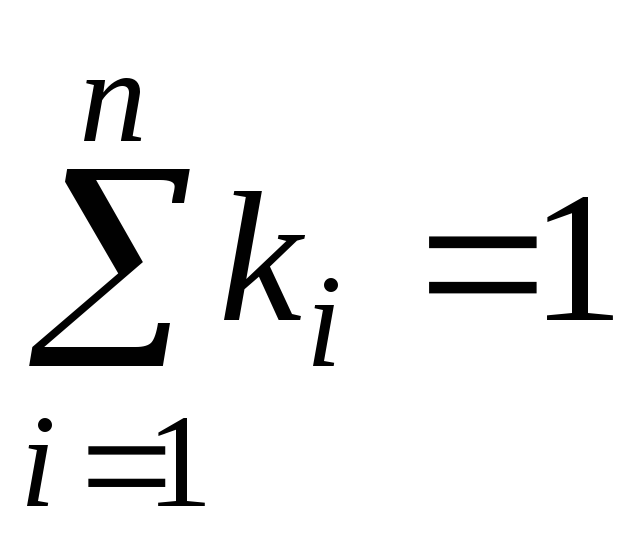 ,
,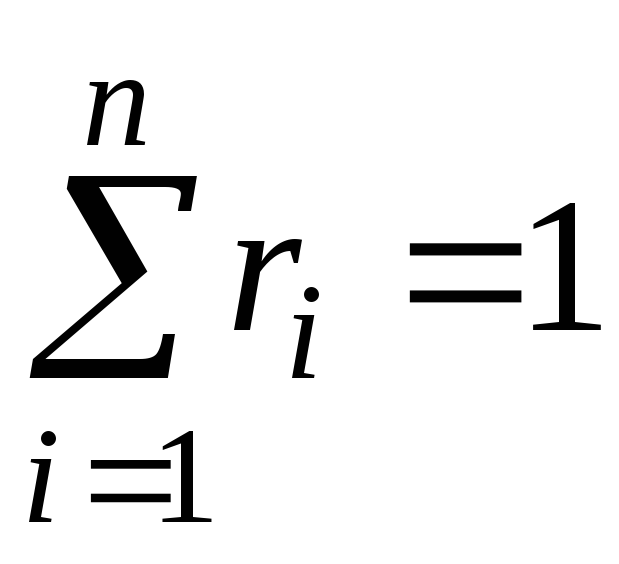 . (11)
. (11)
Often, in practice, the chemical composition of the mixture is not set in fractions i th component, and its percent.
For example, in heat engineering, it is roughly assumed that dry air consists of 79 volume percent nitrogen and 21 volume percent oxygen.
Percent i The th component in the mixture is calculated by multiplying its fraction by 100.
For an example with dry air, we will have:
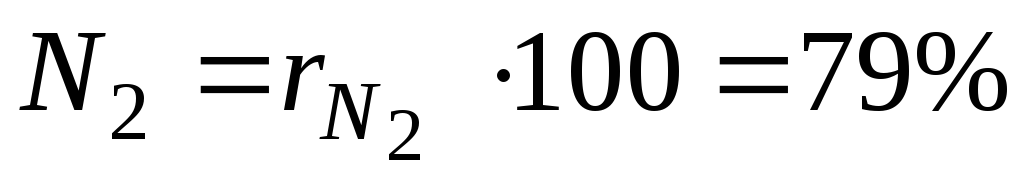 ,
,
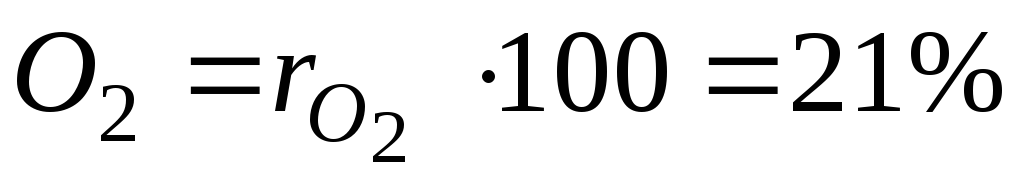 . (12)
. (12)
where 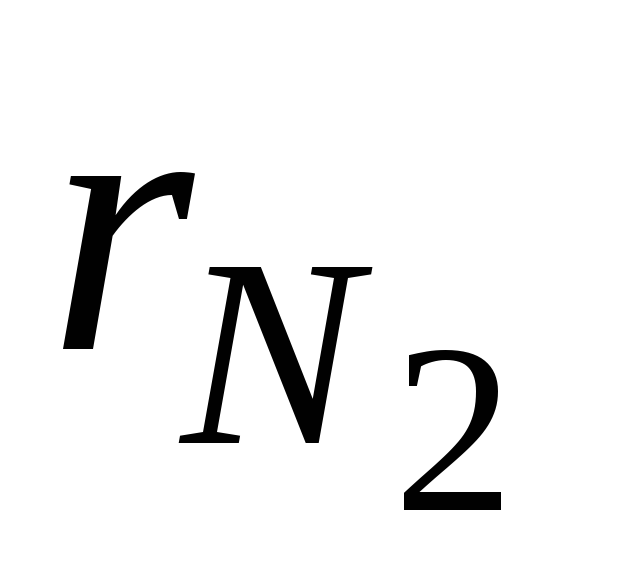 and
and 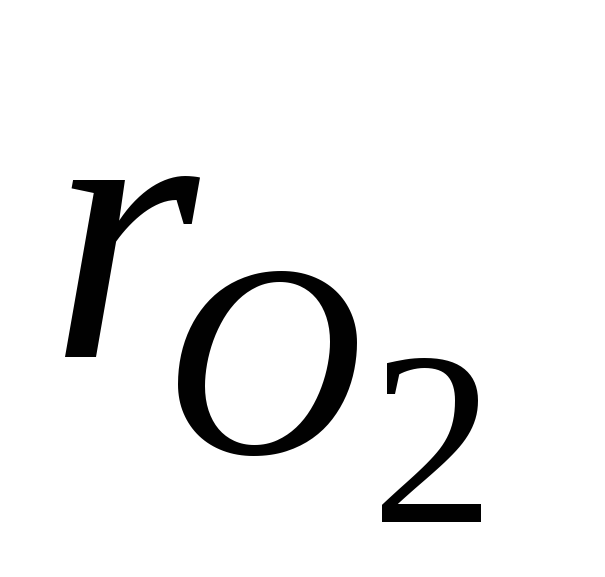 - volume fractions of nitrogen and oxygen in dry air; N 2 and O 2 - designation of the volume percent of nitrogen and oxygen, respectively,% (vol.).
- volume fractions of nitrogen and oxygen in dry air; N 2 and O 2 - designation of the volume percent of nitrogen and oxygen, respectively,% (vol.).
Note:
1)The molar fractions of an ideal mixture are numerically equal to the volume fractions:k i = r i ... Let's prove it.
Using the definition of the volume fraction(10)and Amag's law (3) we can write:
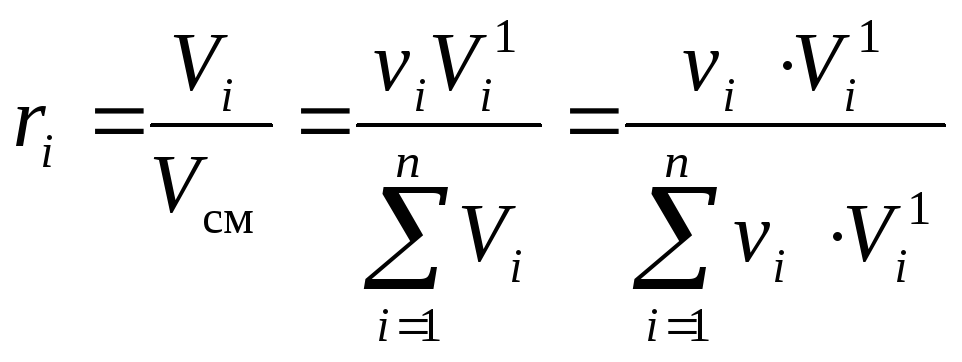 ,
(13)
,
(13)
whereV i - reduced volumeith component, m 3
;
ν
i - number of molesith component, mol;
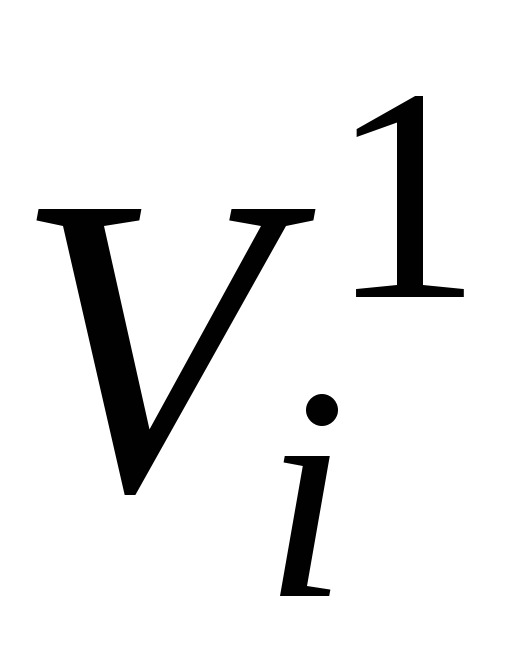 - the volume of one molei-th component at mixture pressure p cm and mixture temperature T cm , m 3
/ mol.
- the volume of one molei-th component at mixture pressure p cm and mixture temperature T cm , m 3
/ mol.
From Avogadro's law (see p. 2.3 of this appendix) it follows that at the same temperature and pressure, one mole of any gas (mixture component) occupies the same volume. In particular, at T cm and p cm it will be some volumeV 1 , m 3 .
The above allows us to write equality:
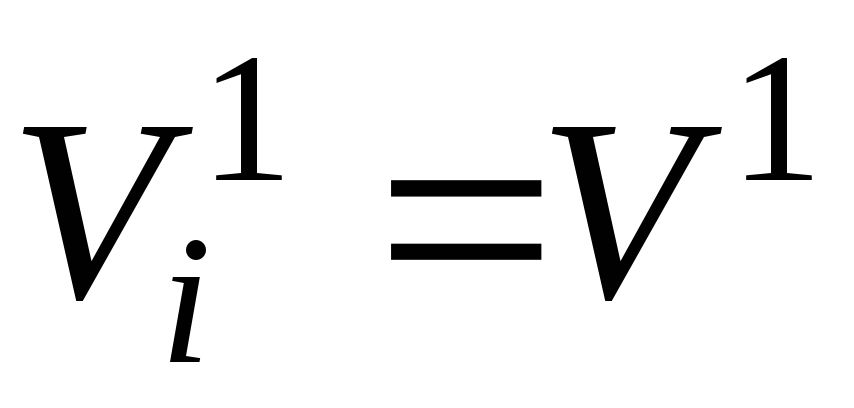 .
(14)
.
(14)
Substituting(14)v(13)we get the required:
![]() .
(15)
.
(15)
2)The volume fractions of the components of the gas mixture can be calculated by knowing their partial pressures. Let's show it.
Consideri-th component of an ideal gas mixture in two different states: when it is at its partial pressure p i ; when it takes up its reduced volumeV i .
The equation of state of an ideal gas is valid for any of its states, in particular, for the two named above.
In accordance with this, and taking into account the definition of the specific volume, we can write down:
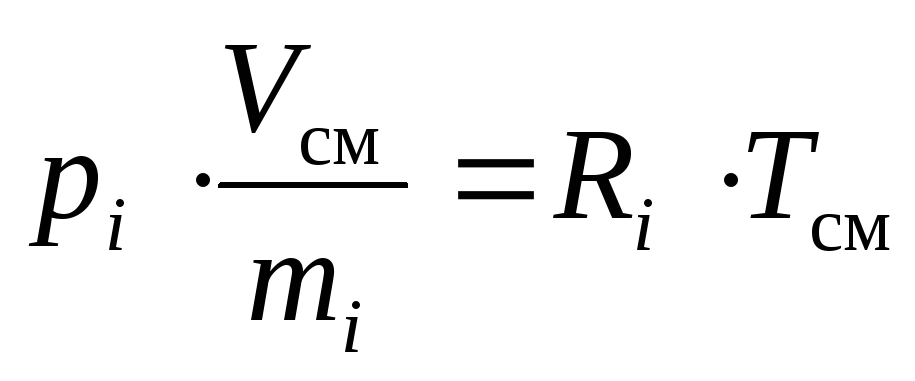 ,
(16)
,
(16)
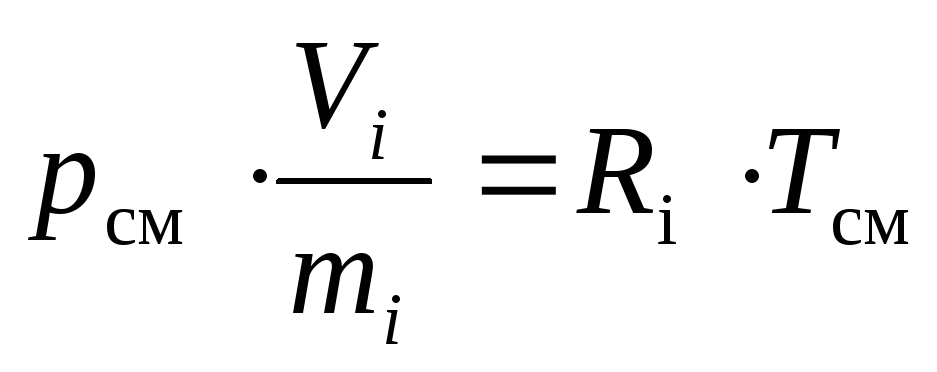 ,
(17)
,
(17)
whereR i - gas constantith component of the mixture, J / (kg · K).
After dividing both parts(16)and(17)on top of each other we get the required:
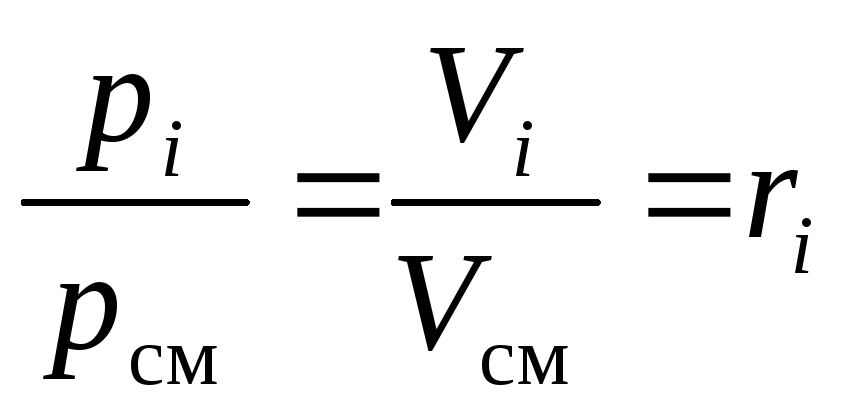 .
(18)
.
(18)
From(18)it can be seen that the partial pressures of the components of the mixture can be calculated from its chemical composition, at a known total pressure of the mixture p cm :
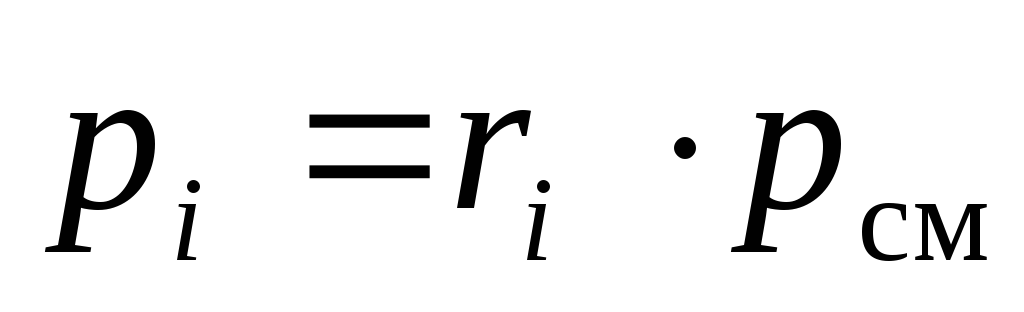 .
(19)
.
(19)