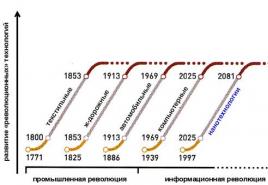
June 23, On the Procedure for calculating the ratio of active and reactive power consumption for individual power receiving devices (groups of power receiving devices) of electrical energy consumers
Even if you listened carefully to everything in your lessons at school and in classes at the university, you don’t know everything interesting facts about chemical elements. In this article we will talk about interesting moments in history associated with chemical elements, as well as their unusual properties.
1. Hydrogen
IN earth's crust Hydrogen is contained very little - about 0.15 percent, while this same element makes up about 50% of the mass of the Sun. Another interesting thing is that in liquid form hydrogen is the densest substance, and in gaseous form, on the contrary, it is the least dense.
2. Sodium

Sodium (better known as salt) originally had a different name. Until the 18th century, people called this element sodium. For this reason, sodium salts had such a strange name as hydrochloric soda, or soda sulfate. Here in Russia, this name took root thanks to Hermann Hess.
3. Metals

Few people know, but iron can go into gaseous state, for this it needs to be heated up to 50,000 degrees Celsius.
4. Gold

One of the most precious metals that everyone knows - gold, is found in places you didn't know about. So, in a ton of ordinary ocean water there is about 7 mg. In total, there are more than 10 billion tons of this metal in the ocean.
5. Platinum

At first, platinum, due to its similarity with silver, was given a similar name - “silver”. It was much cheaper than silver. Later, when they figured out where this metal could be used, everything changed dramatically. Now platinum is tens of times more expensive than silver.
6. Silver

By the way, about silver - its bactericidal properties were discovered by accident. The Macedonian army was exposed to an epidemic, but it affected only ordinary military personnel; the commanders were healthy. It turned out that everything was connected with the dishes. The bosses had it in silver, and the military had it in tin.
7. Metals in liquid state

There are several metals that are liquid at “room” temperature: mercury, cesium, francium and gallium.
8. Metals and planets

Previously, people knew only 7 metals and the same number of planets, so they divided them “in pairs”. The Moon meant silver, Mars - iron, Mercury was assigned to Mercury, and the Sun, naturally, gold. Jupiter became tin, Venus became copper, and Saturn became lead.
Sand snake. Interesting chemical experiment at home:
Chemistry is a subject that is known to all schoolchildren. Attitudes towards it vary: some people like to watch how reagents behave during various experiments in the classroom, while for others, on the contrary, chemistry only causes boredom. However, not everyone knows interesting facts about this discipline. Let's look at some of them.
Dancing squid
Chemistry is a subject that finds practical application in various areas of life. One of the interesting facts about chemistry comes from a Japanese dish called “dancing squid.” Its highlight is as follows: freshly caught squid is served to the guest’s table, shortly before pouring soy sauce over it. The squid begins to move its tentacles as if dancing. This effect is due to the fact that a chemical reaction occurs in the squid's tentacles, causing the muscles to move.
Skatol
Another interesting fact about chemistry involves a special substance called skatole. This is an organic compound that gives feces its characteristic odor. His colorless crystals can be found in various essential oils, resins, they are also formed during the decomposition of protein. In small doses, this substance has a pleasant floral aroma. Manufacturers often add it to perfumes, cigarettes, and various food essences. Skatole is even found in food.

Poison in alcohol
And the following interesting fact about chemistry will serve as a warning for those who are prone to drinking alcohol. They may contain a very dangerous substance, which in taste and smell is practically indistinguishable from ethyl alcohol. This is methyl alcohol. Small amounts of it can cause blindness. A dose of 30 ml can cause cardiac arrest. In case of methyl alcohol poisoning, the antidote to it is ethyl alcohol. This is explained by the fact that the binding processes of both alcohols directly depend on the enzyme alcohol dehydrogenase. This substance reacts faster with ethanol. As a result of the reaction, the ethanol is exhausted, and most of the methanol remains unbroken, resulting in a smaller amount of poison ending up in the blood.
Rescue Canaries
Many interesting facts about chemistry are also connected with the animal world. For example, it is a widely known fact among miners that canaries are highly sensitive to the smell of methane gas. This feature was always used in the past by mine workers, who always took small birds underground with them. If the canaries stopped singing, this meant that they should immediately go upstairs.

Discovery of antibiotics
Perhaps one of the most famous facts about chemistry is associated with the discovery of antibiotics by A. Fleming in 1928. The scientist conducted one of his ordinary experiments, which were devoted to the human body’s fight against various bacterial infections. He grew cultures called Staphylococcus in test tubes. A scientist accidentally left a test tube with bacteria unattended for several days. At this time, a whole colony of mold fungi grew in it. After this, A. Fleming was able to identify a separate active substance- penicillin.
For the first time in human history, these substances were isolated from wheat flour by the Italian scientist Bartolomeo Beccari in 1728. The scientist’s discovery has since been considered the birth of a whole direction in science - protein chemistry. Let's look at some interesting chemistry facts about proteins:
- Every living organism on our planet contains these substances. Protein makes up about half the dry weight of each organism. For example, in viruses its content ranges from 50 to 95%. In addition, proteins are one of the four main components of living matter (the other three are nucleic acids, carbohydrates, and fats). They occupy a special place in their biological functions.

- About 30% of the proteins in the human body are found in muscle tissue. 20% is found in bones and tendons. Only 10% comes from the skin.
- In total, there are about a thousand different proteins in nature. They enable the life of a wide variety of organisms - from protozoa to humans. In total, proteins provide life to two million types of living organisms.
- Brain is also a protein. If alcohol enters the body nerve cells die. This occurs due to the fact that the protein is denatured when interacting with ethyl alcohol.
Six more interesting facts about chemistry
Let us briefly consider a few more facts from this area that will be of interest to both schoolchildren and adults.
- Record holder among scientists who devoted their research to the discovery chemical elements, is the Swedish explorer Carl Scheele. He discovered fluorine, chlorine, barium, oxygen, manganese, molybdenum, and tungsten.
- The thinnest matter that can be seen by the human eye is a soap bubble. The thickness of tissue paper or, for example, a human hair is thousands of times greater than the thickness of the wall of a soap bubble. Its bursting speed is only 0.001 seconds. For comparison: the speed of a nuclear reaction is 0.000 000 000 000 000 001 sec.
- Iron is durable and hard material, however, even it can melt and turn into gas. This happens at a temperature of 1539 0 C.

- The next interesting fact about chemistry is related to the size of atoms. It is known that these particles are extremely small in size. For example, hydrogen atoms are so small that even if they were placed one after another in the amount of 100 million, the length of such a chain would not exceed 1 cm.
- One ton of ocean water contains only 7 milligrams of gold. However, the total mass of precious metal contained in all waters is quite impressive and amounts to 10 billion tons.
- The most modern passenger aircraft use up to 75 tons of oxygen during their operation. The same amount of this substance is produced by 25,000-50,000 hectares of forest during photosynthesis.
First discovered in 1817 and used as a zinc impurity, cadmium was relatively unremarkable until the early 1900s, when zinc mining began at the Kamioka Mine in central Japan. During the zinc purification process, cadmium was discharged into the Jinzu River. By 1930, the waste had affected the bones of local residents and made them incredibly fragile; one doctor broke a girl's wrist while trying to take her pulse. It was not until 1961 that it was determined that cadmium was the cause of this disease. Research showed that local crops were laden with cadmium, which was carried into the rice fields from river water.
The atomic structure of cadmium allows it to bind metallothionein, a protein in the body's cells that binds more biologically important metals. When local residents ate rice, cadmium curtailed zinc, calcium and others minerals necessary to strengthen bones. In 1972, the mining company paid compensation to 178 surviving residents who lived or worked along the river. Twelve years later, when filmmakers needed to kill Godzilla in the final sequel, they used cadmium-tipped missiles.
Chemical element Gallium, disappearing spoon
An element for laboratory pranksters, gallium was discovered in 1875 by French chemist Paul Émile François Lecoq de Boisbaudran. Despite solid state at room temperature, the metal melts as early as 84°F. This means you could hypothetically fashion a spoon out of gallium, hand it to a friend to stir his morning coffee, and see his reaction when the spoon disappears into the hot drink. (Despite gallium's low toxicity, your friend shouldn't drink this coffee.) In addition to its use in practical jokes, gallium's ability to withstand a wide range of temperatures in liquid form makes it a convenient replacement for mercury for high-temperature thermometers.
Chemical element Phosphorus, devil's element
One of the key components in modern explosives Phosphorus was first discovered in an unlikely place: urine. In 1669, German alchemist Hennig Brand attempted to create the “philosopher’s stone,” a legendary artifact that could transform metal into gold. The alchemists paid great value the color of the substances, and since the urine was (more or less) similar in color to gold, Brand probably assumed that he could use it to obtain gold.
Brand had no idea that he had made the first discovery of an element since ancient times
After boiling and decomposing a large amount of liquid waste, presumably taken from local alehouses, the alchemist obtained a black paste. He mixed the result with sand, then heated and distilled it, producing a white, waxy substance that glowed faintly in the dark, sometimes even emitting a flame when exposed to air! (Hence the nickname: “Devil Element”). Brand had no idea that he had made the first discovery of an element since ancient times; he only knew that his unappetizing project did not produce the gold he was looking for.
Chemical element Oxygen, the secret of life
While still a boy, Joseph Priestley noticed that spiders sealed in jars eventually died. He knew his captives had run out of air, but what was left in the jar with the dead spider? Years later, while working as a preacher, Priestley is still occupied with this question. Then an idea struck him: what if there were various types air? Priestley's curiosity only increased when he realized that, unlike animals, plants could survive in sealed jars.
To test his theory, he placed mice in a jar with mint sprigs. When his subjects stayed longer in the jar of greens, he concluded that the plants were producing something vital. Priestley later called his discovery "dephlogisticated air," an awkward term that French chemist Antoine Lavoisier replaced with "oxygen" after conducting a series of similar experiments.
In the early 1770s, Priestley shared his observations with his friend Benjamin Franklin, who later wrote, “I hope this will give cause to reconsider the violent destruction of trees which occurs from the opinion that trees may be infected. I am confident, after long observation, that there is nothing unhealthy in the air of the forests.”
Chemical element Seaborgium
After helping to discover 10 elements at Berkeley, including plutonium, americium and curium, chemist Glenn Seaborg wouldn't mind lending his name to one of them. But in 1974, a team from Russia in the city of Dubna announced that they had discovered element 106, several months before the Berkeley team. Turned around cold war for who exactly first discovered this new element and what name it should bear, the Americans wrote it down as Seaborgium.
The International Union of Pure and Applied Chemistry intervened and abolished the name in the early 90s. Backed by powerful chemistry journals, the Americans insisted on keeping the name, and it was officially restored in 1997. The Dubna city team also received its prize: element 105, dubnium. To celebrate his victory, Seaborg was photographed next to the large periodic table, and his element on it, the only one ever publicly named after a living person.
As we know, substances are made up of atoms. A different types atoms are called chemical elements. In this post you will read many interesting facts about chemical elements.
There are significantly fewer chemical elements than different substances. There are only 80 stable elements (the atoms of which do not decay over time), and there are also several radioactive, but long-lived ones that are also found in nature. All the variety of substances is formed due to the fact that atoms are able to connect with each other. Positively charged nuclei of atoms, when brought closer together, attract negatively charged electrons of other atoms and because of this, a stable bond is formed between the atoms.
Atoms of chemical elements differ from each other in the number of protons in the nucleus of the atom. Protons and neutrons are held in the nucleus nuclear forces, but electromagnetic forces try to push the protons away from each other. The more protons in a nucleus, the stronger the repulsion, so nuclei that are too large cannot exist for long. The last chemical element whose atoms are stable is lead (number 82), and the last one that occurs in nature is uranium (number 92). All known elements with high numbers were obtained artificially in nuclear reactors or accelerators. The heaviest element to date that has been obtained artificially is ununoctium (number 118). It was synthesized by Russian scientists at an accelerator in Dubna. All elements numbered 100 and above are obtained in very small quantities (sometimes only in quantities of a few atoms).
According to modern concepts, all elements heavier than hydrogen and helium were formed during the evolution of stars. The nuclei of atoms from hydrogen to iron are capable of merging with each other, releasing energy, and are gradually formed during the life of the star. But all the chemical elements whose atoms are heavier than iron, according to scientists, were formed during the explosions of supernovae or neutron stars.
The very first chemical element is hydrogen. It is the most common in the Universe, more than 90% of atoms are hydrogen atoms. But there is not much hydrogen on Earth, and the most common element is oxygen. The earth's crust contains about 50% oxygen, followed by silicon (26% by mass) and aluminum (7%).
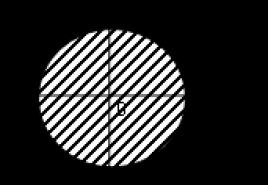
Even pure chemical elements can exist in the form of different substances, since the atoms in them can be combined in different ways. This phenomenon is called allotropy.

example of allotropy - crystalline boron (left) and amorphous boron
Chemical elements differ greatly from each other in their ability to enter into chemical reactions. The most chemically passive elements are - inert gases, especially helium. This is because their outer electron shell is completely filled. Helium and neon do not form real chemical compounds. Also characterized by low chemical activity are the so-called. noble metals - gold, silver, platinum and platinum group metals.
The most active chemical elements are those that easily give up or gain electrons. Most active metal- cesium, and the most active non-metal is fluorine.
Cesium is so active that it spontaneously ignites in air and explodes in water.
video - reaction of cesium with water (first rubidium is thrown into the water, and then cesium)
Fluorine is so active that it reacts with almost all known substances. Even substances such as sand and water ignite in this gas. Fluorine is so dangerous that many chemists, trying to obtain it in its pure form, died during experiments.
video - burning of asbestos and water in fluorine
video - even a brick catches fire in fluorine
Of all the chemical elements in their pure form, 11 elements under normal conditions are gases, and almost all the rest are solids. Only mercury and bromine are liquids.
In their properties, many chemical elements are somewhat similar to each other. For example, among them there are such groups as alkali metals, halogens, inert gases, etc. At the same time, almost any known chemical element is unique in some way and in some areas of application is irreplaceable. For example, titanium, on which super-strong alloys are made, is indispensable in aircraft construction. Silicon is indispensable in microelectronics. Lithium is indispensable in the production of compact batteries. Cesium is indispensable as a material for infrared sensors. Uranium is indispensable in the nuclear industry.
The human body consists of more than 30 chemical elements, without which it cannot function normally. For example, bones are made of calcium compounds, iron is part of blood hemoglobin, iodine is needed for the synthesis of thyroid hormones, etc.
An amazing world is around us, a lot of interesting things surround a person, a lot of things he has no idea about, it’s enough just to remember interesting facts about chemistry and understand what a wonderful world a person lives in.
- Just remember gallium and the effect of a dissolving teaspoon immediately comes to mind.. Surprisingly, at room temperature this metal is similar to aluminum. It begins to melt at 28 degrees Celsius. Scientists chemists often joke about their comrades. They give them hebbled spoons, and then see the surprise of those who come when the metal device simply begins to “melt” in a mug of freshly brewed tea.
- Mercury in a thermometer remains liquid at room temperature.

- Everyone knows the fact that Mendeleev dreamed of the periodic table of chemical elements in a dream. But few people know that the scientist himself, when it came to his table, always said: “I worked on it for maybe twenty years, and you think that I sat down... and it just appeared.”
- Sometimes knowledge of chemistry helps to successfully fight wars. Suffice it to recall the example of a virtually unknown battle of the First World War. This battle was related to the extraction of the metal molybdenum. This metal was used in the construction of the legendary German "Big Bertha" cannon. It was used for a reason; this metal turned out to be so strong that the manufactured barrel, which was fired for several kilometers, was not deformed by the shells from overheating. The only place where molybdenum was mined was in the Colorado mine. Having learned this fact, a group from the German company Krupp, located in those places, took possession of this mine with a fight. The German army was supplied with such durable metal. The Allies did not attach any importance to this skirmish, and only towards the end of the war they realized how thoughtful this strategic move was.

- It is impossible to find water in its original pure form (H2O) in nature.. Water absorbs everything it encounters on its way. Thus, after drinking well water, we consume a “compote”, the composition of which no other person could replicate.

- Water reacts to the world around us
. Scientists used water from the same source in different containers. Classical music was played next to one, and the other was placed in a room with people swearing. As a result, based on the composition and structure of the water, it was possible to determine which container with liquid was located where.

- A mixture of bitter, sweet and sour is exactly how you can describe the taste of grapefruit. After processing 100 liters of this juice, scientists were able to isolate mercaptan. He is a taste record holder. A person can feel the taste of such a compound already at a concentration of 0.02 ng/l. To obtain such a concentration, it is enough to dilute only 2 mg of mercaptan for a tanker of water of 100,000 tons.

- An interesting process can be observed in the symbiosis of the fig tree and fig wasps that live in the fruits of this tree. A ripe berry increases the concentration of carbon dioxide by 10%. This is enough to put female wasps to sleep. The males remain active, fertilize the females and fly away, making a hole in the fruit. CO2 comes out, the awakened females fly away and take the pollen with them.

- The scientific name for oxygen is dephlogisticated air..

- Air is 4/5 nitrogen. If you get into a chamber with nitrogen, such chambers are found, for example, in mines, a person finds himself trapped. Nitrogen is colorless and odorless; it seems to a person that he continues to breathe, not realizing that in a few seconds he will fall dead from lack of air.

- Interesting facts are also found in the lives of great chemists. For example, in 1921, two young men came to the famous artist Dmitry Kustodiev and asked him to paint their portraits. Their desire was not without reason, Kustodiev painted exclusively famous people at that time, and the young men were sure that this is exactly what they would become in the future, even though they were still unknown to anyone. The artist agreed, and the payment was a bag of millet and a rooster. The young people turned out to be Nikolai Simenov and Pyotr Kapitsev, who later became great scientists and laureates Nobel Prize in physics and chemistry.

- Great chemist unknown to anyone. One day, King Gustav III of Sweden visited Paris. French scientists came to him for an audience and began to admire the work of the great Swedish chemist Carl Wilhelm Scheele. The king was happy, but did not understand who he was talking about, and ordered Scheel to be elevated to knighthood. But the Prime Minister also did not know such a person, and by chance another Scheele, an artilleryman, was elevated to this rank. The chemist remained an unknown chemist to everyone.