Chemical composition of the cell. Minerals and their role in the cell
Minerals and their role in the cell
1. What substances are called mineral?
2. What process is called dissociation?
3. What are ions?
Minerals of the cell.
Most of the minerals cells is in the form of salts, dissociated into ions, or in the solid state.
According to their reaction, solutions can be acidic, basic or neutral. The acidity or basicity of a solution is determined by the concentration of H+ ions in it. This concentration is expressed using a hydrogen indicator - pH (“pH”). A neutral reaction of a liquid corresponds to pH = 7.0, an acidic reaction - pH< 7,0 и основной - рН >7.0. The length of the pH scale is from 0 to 14.0.
The pH value in cells is approximately 7.0. Changing it by one or two units is detrimental to the cell.
The constant pH in cells is maintained due to the buffering properties of their contents.
A buffer solution is a solution containing a mixture of a weak acid and its soluble salt. When acidity (the concentration of H+ ions) increases, the free anions that come from the salt readily combine with the free H+ ions and remove them from solution. When acidity decreases, additional H+ ions are released. This way, a relatively constant concentration of H+ ions is maintained in the buffer solution.
Some organic compounds, in particular proteins, also have buffering properties.
Being components of the body's buffer systems, ions determine their properties - the ability to maintain pH at a constant level (close to a neutral reaction), despite the fact that acidic and alkaline products are continuously formed during the metabolic process. So, the phosphate buffer system mammals, consisting of HPO|42- and H2PO-4, maintains the pH of the intracellular fluid within 6.9-7.4. The main buffer system of the extracellular environment (blood plasma) is the bicarbonate system, consisting of H2CO3 and HCO4- and maintaining the pH at 7.4.
Compounds of nitrogen, phosphorus, calcium and other inorganic substances are used for the synthesis of organic molecules (amino acids, proteins, nucleic acids and etc.).
Ions of some metals (Mg, Ca, Ze, Cu, Mn, Mo, Br, Co) are components of many enzymes, hormones and vitamins or activate them. For example, the Fe ion is part of blood hemoglobin, and the Zn ion is part of the hormone insulin. With their deficiency, the most important processes of cell life are disrupted.
Buffer system.
1. In what form are minerals present in living organisms?
2. What is the role of inorganic ions in the cell?
3. What is the role of ions in the body’s buffer systems?
4. Why does the lack or absence of certain metal ions lead to disruption of cell functioning?
Inorganic acids and their salts play an important role in the life of organisms. Thus, hydrochloric acid is part of the gastric juice and creates conditions for the digestion of food proteins. Residues of sulfuric acid help remove water-insoluble substances from the body.
Kamensky A. A., Kriksunov E. V., Pasechnik V. V. Biology 10th grade
Submitted by readers from the website
1). They play the role of cofactors in enzymatic reactions. Thus, many ions form complexes with proteins, including enzymes. For the full manifestation of their catalytic activity, the latter require the presence of mineral cofactors - potassium, calcium, sodium, magnesium, and iron ions. Iron, copper and especially magnesium ions are necessary for the activation of enzymes associated with the transfer and release of energy, transport and oxygen binding.
2). They take part in maintaining osmotic pressure and acid-base balance (phosphate and bicarbonate buffers).
3). Provides blood clotting processes
4). Create membrane potential and action potential of excitable cells
5). Minerals are included in the structures of various organs of the body. Inorganic substances can be in the form of insoluble compounds in the body (for example, in bone and cartilage tissue).
6). Participate in redox reactions, etc.
Sodium and potassium ions play a major role in mineral metabolism. These cations determine the pH value, osmotic pressure, and volume of body fluids. They participate in the formation of bioelectric potentials and in the transport of amino acids, sugars and ions across the cell membrane. Sodium makes up 93% of all blood plasma cations; its concentration in blood plasma is 135-145 mmol/l. Potassium is mainly an intracellular cation; in blood plasma its concentration is 3.3-4.9 mmol/l.
The body of a healthy person weighing about 70 kg contains 150-170 g of sodium. Of these, 25-30% are part of the bones and do not directly participate in metabolism. About 70% of the total sodium in the body is actually exchangeable sodium.
The daily diet of residents of civilized countries contains on average 10-12 g of sodium chloride, but the true human need for it is much lower and approaches 4-7 g. This amount of sodium chloride is contained in ordinary food, which casts doubt on the need for additional salting.
Excessive intake of table salt can lead to an increase in the volume of body fluids, increasing the load on the heart and kidneys. Under these conditions, the increase in the penetration of sodium, and with it water, into the intercellular spaces of the tissues of the walls of blood vessels contributes to their swelling and thickening, as well as the narrowing of the lumen of blood vessels.
The constancy of the content of sodium and potassium ions in the blood plasma is maintained mainly by the kidneys. With a decrease in sodium concentration and an increase in potassium, sodium reabsorption increases and potassium reabsorption decreases, and potassium secretion in the renal tubules increases under the influence of the adrenal cortex mineralocorticoid aldosterone.
The body of a healthy person weighing 70 kg contains 45-35 mmol/kg of potassium. Of these, only 50-60 mmol are in the extracellular space, and the rest of the potassium is concentrated in the cells. Thus, potassium is the main intracellular cation. With age, the total potassium content in the body decreases.
Daily potassium intake is 60-100 mmol; Almost the same amount is excreted by the kidneys and only a little (2%) is excreted in the feces.
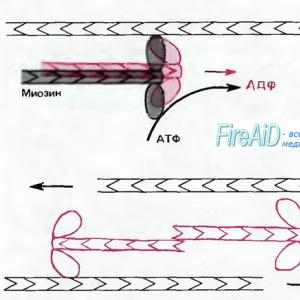
The physiological role of potassium is its participation in all types of metabolism, in the synthesis of ATP and therefore it affects contractility. Its deficiency causes atony of skeletal muscles, a moderate excess causes an increase in tone, and a very high content paralyzes the muscle fiber. Potassium causes vasodilation. It is also involved in the synthesis of acetylcholine, in the destruction of cholinesterase and, therefore, affects the synaptic transmission of excitation. Together with other ions, it provides the cell with the ability to excite.
Chlorine is the second extracellular anion after sodium. Its concentration in extracellular fluid and plasma is 103-110 mmol/l. The total chlorine content in the body is about 30 mmol/kg. A significant amount of chlorine was found only in the cells of the gastric mucosa. It is this that is the reserve for the synthesis of hydrochloric acid in gastric juice, combining with hydrogen ions, which are extracted from the blood by the cells of the mucous membrane and removed into the lumen of the stomach.
Normal plasma calcium levels are 2.1-2.6 mmol/l. Of these, 50% are associated with plasma proteins (especially albumin), 10% are part of soluble complexes, 40% are in free ionized form, which is of greatest interest from a clinical point of view.
Only free Ca 2+ ions are physiologically active, therefore the regulation of metabolism is aimed at maintaining a constant plasma concentration not of total calcium, but only of its physiologically active fraction.
Calcium ions bound to phosphorus ions have the greatest functional activity. Calcium takes an active part in the processes of excitation, synaptic transmission, muscle contraction, cardiac activity, participates in the oxidative phosphorylation of carbohydrates and fats, in blood clotting, affects the permeability of cell membranes, and forms the structural basis of the bone skeleton. A significant portion of intracellular calcium is located in the endoplasmic reticulum (T tanks).
The main role in regulating the balance between plasma calcium and bone calcium belongs to the hormone of the parathyroid glands (parathyrin).
When food containing significant amounts of calcium is consumed, most of it is excreted through the intestines as a result of precipitation in the main intestinal environment in the form of insoluble compounds.
Phosphorus enters the body mainly with dairy, meat, fish and legume products. Its concentration in blood serum is 0.81-1.45 mmol/l. The daily requirement for phosphorus is approximately 1.2 g, in pregnant and lactating women - up to 1.6-1.8 g. Phosphorus is an anion of intracellular fluid, high-energy compounds, coenzymes of tissue respiration and glycolysis. Insoluble calcium phosphates constitute the major mineral component of bones, giving them strength and hardness. Salts of phosphoric acid and its esters are components of buffer systems for maintaining the acid-base state of tissues.
Iron is necessary for oxygen transport and for oxidative reactions, as it is part of hemoglobin and mitochondrial cytochromes. Its concentration in the blood in combination with the transport protein transferrin is normally 1.0-1.5 mg/l. The daily requirement for iron for men is 10 mg; for women of childbearing age, due to menstrual blood loss, this value is much higher and approaches 18 mg. For pregnant and lactating women, due to the needs of the child’s body, this parameter approaches 33 and 38 mg, respectively. Iron is found in meat, liver, legumes, buckwheat and millet cereals. Insufficient iron intake in the body is common. Thus, 10-30% of women of childbearing age have iron deficiency anemia.
Iodine is the only known trace element involved in the construction of hormone molecules. Sources of iodine are sea plants and sea fish, meat and dairy products. The iodine concentration in blood plasma is 10-15 mcg/l. The daily requirement is 100-150 mcg, for pregnant and lactating women - 180-200 mcg. Up to 90% of organic iodine circulating in the blood comes from thyroxine and triiodothyronine. Insufficient intake of iodine in the body can cause dysfunction of the thyroid gland.
Fluoride protects teeth from caries. The daily need for fluoride is 0.5-1.0 mg. It enters the body with drinking water, fish, nuts, liver, meat, and oat products. It is believed that it blocks microelements necessary for the activation of bacterial enzymes. Fluoride stimulates hematopoiesis, immune reactions, and prevents the development of senile osteoporosis.
Magnesium is an intracellular cation (Mg 2+), contained in the body in an amount of 30 mmol/kg body weight. The concentration of magnesium in blood plasma is 0.65-1.10 mmol/l. The daily requirement for it is about 0.4 g. Magnesium is a catalyst for many intracellular processes, especially those related to carbohydrate metabolism. It reduces the excitability of the nervous system and the contractile activity of skeletal muscles, helps to dilate blood vessels, reduce heart rate and lower blood pressure.
Inorganic ions, or minerals, perform the following functions in the body:
1. Bioelectric function. This function is associated with the occurrence of a potential difference on cell membranes. The ion concentration gradient on both sides of the membrane creates a potential of about 60-80 mV in different cells. The inner side of the cell membrane is negatively charged relative to the outer. The higher the electrical potential of the membrane, the higher the protein content and its ionization (negative charge) inside the cell and the concentration of cations outside the cell (the diffusion of Na + and K + ions through the membrane into the cell is difficult). This function of inorganic ions is used to regulate the functions of especially excitable cells (nerve, muscle) and to conduct nerve impulses.
2. Osmotic function used to regulate osmotic pressure. A living cell obeys the law of isosmopolarity: in all environments of the body, between which there is a free exchange of water, the same osmotic pressure is established. If the number of ions in a certain medium increases, then water rushes after them until a new equilibrium and a new level of osmotic pressure are established.
3. Structural function due to the complexing properties of metals. Metal ions interact with anionic groups of proteins, nucleic acids and other macromolecules and thereby ensure, along with other factors, the maintenance of certain conformations of these molecules. Since the biological activity of biopolymers depends on their conformations, the normal implementation of their functions by proteins, the unhindered implementation of information contained in nucleic acids, the formation of supramolecular complexes, the formation of subcellular structures and other processes are unthinkable without the participation of cations and anions.
4. Regulatory function is that metal ions are enzyme activators and thereby regulate the rate of chemical transformations in the cell. This is a direct regulatory effect of cations. Indirectly, metal ions are often necessary for the action of another regulator, for example, a hormone. Let's give a few examples. The formation of the active form of insulin is impossible without zinc ions. The tertiary structure of RNA is largely determined by the ionic strength of the solution, and cations such as Cr 2+, Ni 2+, Fe 2+, Zn 2+, Mn 2+ and others are directly involved in the formation of the helical structure of nucleic acids. The concentration of Mg 2+ ions affects the formation of such a supramolecular structure as ribosomes.
5. Transport function manifests itself in the participation of certain metals (as part of metalloproteins) in the transfer of electrons or simple molecules. For example, iron and copper cations are part of cytochromes, which are carriers of electrons in the respiratory chain, and iron in hemoglobin binds oxygen and participates in its transfer.
6. Energy function associated with the use of phosphate anions in the formation of ATP and ADP (ATP is the main carrier of energy in living organisms).
7. Mechanical function. For example, the Ca +2 cation and phosphate anion are part of the hydroxylapatite and calcium phosphate of bones and determine their mechanical strength.
8. Synthetic function. Many inorganic ions are used in the synthesis of complex molecules. For example, iodine ions I¯ are involved in the synthesis of iodothyronines in thyroid cells; anion (SO 4) 2- - in the synthesis of ester-sulfur compounds (during the neutralization of harmful organic alcohols and acids in the body). Selenium is important in the mechanism of protection against the toxic effects of peroxide. It forms selenocysteine, an analogue of cysteine, in which selenium atoms replace sulfur atoms. Selenocysteine is a component of the enzyme glutathione peroxidase, which catalyzes the reduction of hydrogen peroxide with glutathione (tripeptide - γ-glutamyl-cysteinylglycine)
It is important to note that, within certain limits, interchangeability of some ions is possible. If there is a deficiency of a metal ion, it can be replaced by another metal ion that is similar in physicochemical properties and ionic radius. For example, the sodium ion is replaced by a lithium ion; calcium ion - strontium ion; molybdenum ion - vanadium ion; iron ion - cobalt ion; sometimes magnesium ions - manganese ions.
Due to the fact that minerals activate the action of enzymes, they affect all aspects of metabolism. Let us consider how the metabolism of nucleic acids, proteins, carbohydrates and lipids depends on the presence of certain inorganic ions.
From this lesson you will learn about the role of mineral compounds of micro- and macroelements in the life of living organisms. You will get acquainted with the hydrogen indicator of the environment - pH, learn how this indicator is related to the physiology of the body, how the body maintains a constant pH of the environment. Find out the role of inorganic anions and cations in metabolic processes, learn details about the functions of Na, K and Ca cations in the body, as well as what other metals are part of our body and what their functions are.
Introduction
Topic: Basics of cytology
Lesson: Minerals and their role in cell life
1. Introduction. Minerals in the cell
Minerals constitute from 1 to 1.5% of the wet weight of the cell, and are found in the cell in the form of salts dislocated into ions, or in the solid state (Fig. 1).
Rice. 1. Chemical composition of cells of living organisms
In the cytoplasm of any cell there are crystalline inclusions, which are represented by slightly soluble calcium and phosphorus salts; In addition to them, there may be silicon oxide and other inorganic compounds that participate in the formation of supporting structures of the cell - in the case of the mineral skeleton of radiolarians - and the body, that is, they form the mineral substance of bone tissue.
2. Inorganic ions: cations and anions
Inorganic ions are important for the life of the cell (Fig. 2).

Rice. 2. Formulas of the main ions of the cell
Cations- potassium, sodium, magnesium and calcium.
Anions- chloride anion, bicarbonate anion, hydrogen phosphate anion, dihydrogen phosphate anion, carbonate anion, phosphate anion and nitrate anion.
Let's consider the meaning of ions.
Ions, located on opposite sides of cell membranes, form the so-called transmembrane potential. Many ions are unevenly distributed between the cell and the environment. Thus, the concentration of potassium ions (K+) in the cell is 20-30 times higher than in the environment; and the concentration of sodium ions (Na+) is ten times lower in the cell than in the environment.
Thanks to existence concentration gradients, many vital processes are carried out, such as contraction of muscle fibers, excitation of nerve cells, and transfer of substances across the membrane.
Cations affect the viscosity and fluidity of the cytoplasm. Potassium ions reduce viscosity and increase fluidity, calcium ions (Ca2+) have the opposite effect on the cell cytoplasm.
Anions of weak acids - bicarbonate anion (HCO3-), hydrogen phosphate anion (HPO42-) - are involved in maintaining the acid-base balance of the cell, that is pHenvironment. According to their reaction, solutions can be sour, neutral And main.
The acidity or basicity of a solution is determined by the concentration of hydrogen ions in it (Fig. 3).

Rice. 3. Determination of the acidity of a solution using a universal indicator
This concentration is expressed using the pH indicator, the length of the scale is from 0 to 14. Neutral medium pH is about 7. Acidic medium is less than 7. Basic medium is more than 7. You can quickly determine the pH of the medium using indicator papers or strips (see video) .
We dip the indicator paper into the solution, then remove the strip and immediately compare the color of the indicator zone of the strip with the colors of the standard comparison scale that is included in the kit, assessing the similarity of the color and determining the pH value (see video).
3. pH of the environment and the role of ions in its maintenance
The pH value in the cell is approximately 7.
A change in pH in one direction or another has a detrimental effect on the cell, since the biochemical processes taking place in the cell immediately change.
The constancy of the cell pH is maintained thanks to buffer properties its contents. A buffer solution is a solution that maintains a constant pH value. Typically, a buffer system consists of a strong and weak electrolyte: a salt and a weak base or weak acid that form it.
The effect of a buffer solution is that it resists changes in the pH of the environment. A change in the pH of the medium can occur as a result of concentrating the solution or diluting it with water, acid or alkali. When acidity, that is, the concentration of hydrogen ions, increases, free anions, the source of which is the salt, interact with protons and remove them from the solution. When acidity decreases, the tendency to release protons increases. In this way, the pH is maintained at a certain level, that is, the concentration of protons is maintained at a certain constant level.
Some organic compounds, in particular proteins, also have buffering properties.
Cations of magnesium, calcium, iron, zinc, cobalt, manganese are part of enzymes and vitamins (see video).
Metal cations are part of hormones.
Zinc is part of insulin. Insulin is a pancreatic hormone that regulates blood glucose levels.
Magnesium is part of chlorophyll.
Iron is part of hemoglobin.
With a lack of these cations, the vital processes of the cell are disrupted.
4. Metal ions as cofactors
The importance of sodium and potassium ions
Sodium and potassium ions are distributed throughout the body, while sodium ions are mainly included in the intercellular fluid, and potassium ions are contained inside cells: 95% of ions potassium contained inside cells, and 95% of ions sodium contained in intercellular fluids(Fig. 4).

Associated with sodium ions osmotic pressure fluids, tissue water retention, and transport, or transport substances such as amino acids and sugars through the membrane.
The importance of calcium in the human body
Calcium is one of the most abundant elements in the human body. The bulk of calcium is found in bones and teeth. The fraction outside bone calcium makes up 1% of the total amount of calcium in the body. Extraosseous calcium affects blood clotting, as well as neuromuscular excitability and muscle fiber contraction.
Phosphate buffer system
The phosphate buffer system plays a role in maintaining the acid-base balance of the body; in addition, it maintains the balance in the lumen of the kidney tubules, as well as intracellular fluid.
The phosphate buffer system consists of dihydrogen phosphate and hydrogen phosphate. Hydrogen phosphate binds, that is, neutralizes the proton. Dihydrogen phosphate releases a proton and interacts with alkaline products entering the blood.
The phosphate buffer system is part of the blood buffer system (Fig. 5).

Blood buffer system
In the human body, there are always certain conditions for a shift in the normal reaction of the tissue environment, for example, blood, towards acidosis (acidification) or alkalosis (deoxidation - an upward shift in pH).
Various products enter the blood, for example, lactic acid, phosphoric acid, sulfurous acid, formed as a result of the oxidation of organophosphorus compounds or sulfur-containing proteins. In this case, the blood reaction may shift towards acidic foods.
When eating meat products, acidic compounds enter the blood. When eating plant foods, bases enter the blood.
However, the pH of the blood remains at a certain constant level.
There are in the blood buffer systems, which maintain pH at a certain level.
Blood buffer systems include:
Carbonate buffer system,
Phosphate buffer system,
Hemoglobin buffer system,
Plasma protein buffer system (Fig. 6).
The interaction of these buffer systems creates a certain constant pH of the blood.

Thus, today we looked at minerals and their role in the life of the cell.
Homework
What chemicals are called minerals? What is the importance of minerals for living organisms? What substances do living organisms mainly consist of? What cations are found in living organisms? What are their functions? What anions are found in living organisms? What is their role? What is a buffer system? What blood buffer systems do you know? What is the content of minerals in the body related to?
1. Chemical composition of living organisms.
2. Wikipedia.
3. Biology and medicine.
4. Educational center.
Bibliography
1. Kamensky A. A., Kriksunov E. A., Pasechnik V. V. General biology 10-11 grade Bustard, 2005.
2. Biology. Grade 10. General biology. Basic level / P. V. Izhevsky, O. A. Kornilova, T. E. Loshchilina and others - 2nd ed., revised. - Ventana-Graf, 2010. - 224 pp.
3. Belyaev D.K. Biology 10-11 grade. General biology. A basic level of. - 11th ed., stereotype. - M.: Education, 2012. - 304 p.
4. Agafonova I. B., Zakharova E. T., Sivoglazov V. I. Biology 10-11 grade. General biology. A basic level of. - 6th ed., add. - Bustard, 2010. - 384 p.
1. What substances are called mineral?
Answer. Minerals are chemical elements necessary for a living organism to ensure normal functioning (calcium, phosphorus, potassium, magnesium)
Magnesium is a vital element; its participation helps muscles relax. Magnesium inhibits the excitation of nerve endings, participates in many catalytic processes, has the ability to stimulate intestinal motility, thereby promoting the removal of toxins (including cholesterol) and increasing the secretion of bile. Magnesium has a vasodilating effect and improves blood supply to the heart muscle.
Potassium is a mineral that is necessary for the normal functioning of cells of the peripheral and central nervous system, to maintain osmotic pressure, and for the normal functioning of all muscles. They help remove water from the body, and therefore harmful metabolic products.
Sodium. Table salt is necessary for our body. It is a component of blood and tissue fluid. The necessary amount enters the body with food.
Phosphorus is an essential element that is part of nucleic acid proteins and bone tissue; it affects growth and restoration processes in tissues. Phosphorus is needed for bones and is also needed in muscles. The human energy accumulator is adenosine triphosphoric acid (ATP). When a person works, this acid disintegrates, releasing the energy contained in it.
A vital element is sulfur, the significance of which is primarily determined by the fact that it is included in proteins in the form of sulfur-containing amino acids (cysteine and methionine), as well as in the composition of some hormones and vitamins. A person's need for sulfur is satisfied (about 1 g per day) with a normal daily diet.
Chlorine is also a vital element that is involved in the formation of gastric juice, forms plasma, and activates a number of enzymes. The chlorine content in food products ranges from 2-160 mg/%. Without the addition of table salt, the diet would contain 1.6 g of chlorine.
Iron is necessary for hematopoiesis; it ensures the transport of oxygen from the lungs to the tissues. Iron is part of hemoglobin - the red pigment of blood. Red blood cells are produced in the bone marrow; They enter the blood and circulate in it for 6 weeks. They then disintegrate into their component parts, and the iron contained in them enters the spleen and liver, depositing there “until required.”
Zinc is found in blood and muscle tissue. This element is necessary, the significance of which is determined by the fact that it is part of the pancreatic hormone insulin, which regulates blood sugar levels. It is also important for complete wound healing, participates in the regulation of blood pressure and promotes the formation of prostaglandins, which have an anti-inflammatory effect; helps remove cholesterol from the body.
2. What process is called dissociation?
Answer. Electrolytic dissociation is the process of decomposition of an electrolyte into ions when it is dissolved in water or upon melting.
Dissociation into ions occurs due to the interaction of a solute with a solvent; According to spectroscopic methods, this interaction is largely chemical in nature. Along with the solvating ability of solvent molecules, a certain role in electrolytic dissociation is also played by the macroscopic property of the solvent - its dielectric constant
3. What are ions?
Answer. An ion is a particle in which the total number of protons is not equivalent to the total number of electrons. An ion in which the total number of protons is greater than the total number of electrons has a positive charge and is called a cation. An ion in which the total number of protons is less than the total number of electrons has a negative charge and is called an anion.
In the form of independent particles, ions are found in all aggregate states of matter: in gases (in particular, in the atmosphere), in liquids (in melts and solutions), in crystals and in plasma (in particular, in interstellar space).
Questions after §8
1. In what form are minerals present in living organisms?
Answer. Most of the mineral substances of the cell are in the form of salts, dissociated into ions, or in the solid state.
In the cytoplasm of almost any cell there are crystalline inclusions, usually consisting of slightly soluble calcium and phosphorus salts. In addition to them, they may contain silicon dioxide and other inorganic substances. They are used to form supporting structures of the cell (for example, the mineral skeleton of radiolarians) and the body - the mineral substance of bone tissue (calcium and phosphorus salts), mollusk shells (calcium salts), chitin (calcium salts), etc.
2. What is the role of inorganic ions in the cell?
Answer. Inorganic ions, which are of no small importance for ensuring the vital processes of the cell, are represented by cations (K+, Na+, Ca2+, Mg2+, NH) and anions (Cl-, HPO, H2PO, HCO, NO, PO, CO) of mineral salts. The concentration of cations and anions in the cell and in its environment is different. As a result, a potential difference is formed between the contents of the cell and its surrounding environment, providing such important processes as irritability and transmission of excitation along a nerve or muscle.
3. What is the role of ions in the body’s buffer systems?
Answer. The constant pH in cells is maintained due to the buffering properties of their contents. A buffer solution is a solution containing a mixture of a weak acid and its soluble salt. When acidity (the concentration of H+ ions) increases, the free anions that come from the salt readily combine with the free H+ ions and remove them from solution. When acidity decreases, additional H+ ions are released. This way, a relatively constant concentration of H+ ions is maintained in the buffer solution. Some organic compounds, in particular proteins, also have buffering properties.
Being components of the body's buffer systems, ions determine their properties - the ability to maintain pH at a constant level (close to a neutral reaction), despite the fact that acidic and alkaline products are continuously formed during the metabolic process. Thus, the phosphate buffer system of mammals, consisting of HPO42- and H2PO4-, maintains the pH of the intracellular fluid in the range of 6.9–7.4. The main buffer system of the extracellular environment (blood plasma) is the bicarbonate system, consisting of H2CO3 and HCO4- and maintaining a pH of 7.4
4. Why does the lack or absence of certain metal ions lead to disruption of cell functioning?
Answer. Ions of some metals (Mg, Ca, Fe, Zn, Cu, Mn, Mo, Br, Co) are components of many enzymes, hormones and vitamins or activate them. For example, the Fe ion is part of blood hemoglobin, and the Zn ion is part of the hormone insulin. With their deficiency, the most important processes of cell life are disrupted.