How can functional derivatives of carboxylic acids be detected? Abstract: Functional derivatives of carboxylic acids
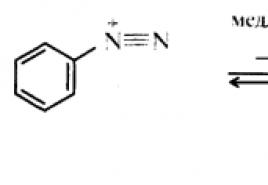
Aromatic diazo compounds.
Reactions of aryldiazonium salts with the release of nitrogen.
Reactions resulting in the diazo group replaced by other groups , have great synthetic application, since they allow, under rather mild conditions, the introduction into the aromatic ring of those functional groups, the introduction of which by other means would be associated with significant difficulties or is simply impracticable. In addition, using these reactions, it is possible to obtain derivatives of aromatic hydrocarbons with such a mutual arrangement of functions that cannot be achieved using direct electrophilic substitution reactions. Reactions that release nitrogen can occur by ionic or radical mechanisms .
Replacing a diazo group with a hydroxyl group. When aqueous solutions of aryldiazonium salts are heated, even to room temperature, nitrogen is released and the corresponding compounds are formed phenols . In many cases, the yields in this reaction are high, so it can serve as a preparative method for the production of phenols. To avoid replacement of the diazo group by other nucleophiles, the reaction is usually carried out using sulfuric acid , the anions of which have low nucleophilicity:
The reaction proceeds according to the mechanism monomolecular aryl nucleophilic substitution S N 1 Ar which is mainly characteristic of diazonium salts. In the first, slow stage, the diazonium cation reversibly dissociates to form an aryl cation (in particular, a phenyl cation) and a nitrogen molecule. In the second stage, the extremely unstable aryl cation quickly combines with the nucleophile. The instability of the aryl cation is due to the impossibility of participation of the π-electrons of the aromatic ring in the delocalization of the positive charge, since the p-orbitals of the ring cannot interact with the vacant sp 2 hybrid orbital located in the plane of the σ-skeleton:

Replacing the diazo group with fluorine . When dry aryldiazonium borofluorides are heated, aryl fluorides ( Schiemann reaction ) :

This reaction is one of the best ways to introduce fluorine into an aromatic ring. It is believed that it flows through ionic mechanism with the formation of an intermediate aryl cation:
Replacement of diazo group with iodine . When a soluble salt of hydroiodic acid is added to solutions of aryldiazonium salts, the corresponding aryliodides . For example, p-diiodobenzene is obtained from p-phenylenediamine in almost quantitative yield, which is quite difficult to obtain by other methods:
Replacing the diazo group with chlorine or bromine. To obtain chloro- or bromine derivatives, diazonium salts are heated in the presence of copper(I) salts - CuCl or CuBr, respectively:

Both reactions proceed according to radical mechanism . The Cu + ion is easily oxidized to the Cu 2+ ion, donating one electron to the diazonium cation. The latter is converted into a free radical (I), which splits off a nitrogen molecule, forming an aryl radical (II). Upon subsequent interaction of the aryl radical (II) with the halide ion, the final ar is formed yl halide . The electron split off at the last stage is spent on the reduction of the Cu 2+ ion, due to which the catalyst is regenerated.
Replacing a diazo group with a cyano group. When solutions of aromatic diazonium salts are treated with copper cyanide, arylnitriles ( aryl cyanides ):

Replacing a diazo group with a nitro group. The reaction is carried out by adding solid aryldiazonium borofluoride to a solution of sodium nitrite in which copper powder is suspended. This method allows you to introduce a nitro group into positions of the aromatic ring that are inaccessible for direct nitration, for example:
Replacing the diazo group with hydrogen. When aryldiazonium salts are exposed to a reducing agent such as hypophosphorous acid H 3 PO 2 , the diazo group is replaced by a hydrogen atom. As an example, a scheme is given for the preparation of 2,4,6-tribromobenzoic acid, which cannot be obtained by direct bromination of benzoic acid:
Replacing a diazo group with a metal. Organic compounds of some metals can be obtained from diazonium salts. For example, when double mercury salts are reduced with copper, organomercury compounds are obtained ( Nesmeyanov's reaction ):

Functional derivatives of carboxylic acids. Dibasic carboxylic acids. a , b -Unsaturated acids
Carboxylic acid derivatives
1. Acid halides .
When exposed to phosphorus halides or thionyl chloride, the formation of halides occurs:
CH 3 COOH + PCl 5 ® CH 3 COCl + POCl 3 + HCl
The halogen in acid halides is highly reactive. A strong inductive effect determines the ease of substitution of halogen with other nucleophiles: - OH , - OR , - N.H. 2, - N 3, - CN and etc.:
CH 3 COCl + CH 3 COOAg ® (CH3CO)2O acetic anhydride + AgCl
1. Anhydrides.
Anhydrides are formed by the reaction of acid salts with their acid halides:
CH 3 COONa + CH 3 COCl ® NaCl + ( CH 3 CO ) 2 O
Acid anhydrides are highly chemically active and, like acid halides, are good acylating agents.
2. Amides .
Amides are obtained via acid halides
CH 3 COCl +2 NH 3 ® CH 3 CONH 2 acetamide +NH4Cl
or from ammonium salts of acids, during dry distillation of which water is split off and an acid amide is formed. Also, acid amides are formed as a by-product during the hydrolysis of nitriles. Amidation processes are important industrially for the production of a number of valuable compounds ( N , N-dimethylformamide, dimethylacetamide, ethanolamides of higher acids).
4. Nitriles. The most important representatives of nitriles are acetonitrile CH 3 CN(used as a polar solvent) and acrylonitrile CH 2 = CHCN(monomer for the production of synthetic neuron fiber and for the production of divinylnitrile synthetic rubber, which is oil and gasoline resistant). The main method for producing nitriles is the dehydration of amides on acid catalysts:
CH 3 CONH 2 ® CH 3 C - CN + H 2 O
5. Esters. Esters of carboxylic acids are of important practical importance as solvents, hydraulic fluids, lubricating oils, plasticizers and monomers. They are obtained by esterification of alcohols with acids, anhydrides and acid halides or by the reaction of acids and alkenes:
CH 3 -CH=CH 2 + CH 3 COOH ® CH 3 COOCH(CH 3) 2
Many esters are used as aromatic substances:
| CH 3 COOCH 2 CH 3 |
pear essence |
| CH 3 CH 2 CH 2 COOCH 2 CH 2 CH 2 CH 2 CH 3 |
pineapple essence |
| HCOOCH 2 CH 3 |
rum essence |
Dibasic saturated acids
Dibasic saturated (saturated) acids have the general formula CnH 2 n ( COOH ) 2 . Of these, the most important are:
NOOS-SOUN- oxalic, ethanedicarboxylic acid;
NOOS-CH 2 -COOH- malonic, propanedicarboxylic acid;
NOOS-CH 2 -CH 2 -COOH- succinic, butanedicarboxylic acid;
NOOS-CH 2 -CH 2 -CH 2 -COOH- glutaric, pentanedicarboxylic acid.
Methods of obtaining
General methods for producing dibasic acids are similar to methods for producing monobasic acids (oxidation of glycols, hydrolysis of dinitriles, Kolbe synthesis - see Lecture No. 27).
1. Oxidation of hydroxy acids :
OH-CH2CH2COOH ® HOCCH 2 COOH ® HOOC-CH2-COOH
2. Oxidation of cycloalkanes .
This is an industrial method for obtaining adipic acid HOOC - CH 2 CH 2 CH 2 CH 2 - COOH from cyclohexane.
Succinic and oxalic acids are also formed as by-products. Adipic acid is used for fiber synthesis nylon 6.6 and plasticizers.
Chemical properties
Dibasic acids are stronger than monobasic acids. This is explained by the mutual influence of carboxyl groups that facilitate dissociation:

In general, the reactions of dicarboxylic acids and their monocarboxylic analogues are almost the same. The reaction mechanism for the formation of diamides, diesters, etc. from carboxylic acids is the same as for monocarboxylic acids. The exception is dicarboxylic acids, which contain fewer than four carbon atoms between the carboxyl groups. Such acids, whose two carboxyl groups are capable of reacting with the same functional group or with each other, exhibit unusual behavior in reactions that proceed to form five- or six-membered closed activated complexes or products.
An example of the unusual behavior of carboxylic acids is the reactions that occur when heated.
At 150 o C, oxalic acid decomposes into formic acid and CO 2 :
HOOC-COOH ® HCOOH + CO2
2. Cyclodehydration .
When heated g-dicarboxylic acids, in which the carboxyl groups are separated by carbon atoms, undergo cyclodehydration, resulting in the formation of cyclic anhydrides:

3. Syntheses based on malonic ester .
Dibasic acids with two carboxyl groups on one carbon atom, i.e. malonic acid and its mono- and disubstituted homologues, when heated slightly above their melting temperatures, decompose (are subjected to decarboxylation) with the elimination of one carboxyl group and the formation of acetic acid or its mono- and disubstituted homologues:
HOOCCH 2 COOH ® CH 3 COOH + CO 2
HOOCCH(CH3)COOH ® CH3CH2COOH + CO 2
HOOCC(CH 3) 2 COOH ® (CH3) 2 CHCOOH + CO 2
The hydrogen atoms of the methylene group located between the acyl groups of malonic acid diethyl ester ( malonic ester), have acidic properties and give a sodium salt with sodium ethoxide. This salt sodium malonic ester– alkylate by the mechanism of nucleophilic substitution S N 2 . Based on sodium malonic ester, mono- and dibasic acids are obtained:
-Na++RBr ® RCH(COOCH 2 CH 3) 2 + 2 H 2 O ®
R-CH(COOH)2 alkylmalonic acid ® R-CH2COOH alkylacetic acid +CO2
4. Pyrolysis of calcium and barium salts .
During pyrolysis of calcium or barium salts adipic (C 6), pimeline (C 7) And cork (From 8) acids are eliminated CO 2 and cyclic ketones are formed:

Unsaturated monobasic carboxylic acids
Unsaturated monobasic acids of the ethylene series have the general formula CnH 2 n -1 COOH, acetylene and diethylene series - CnH 2 n -3 COOH. Examples of unsaturated monobasic acids:
Unsaturated monobasic acids differ from saturated ones by large dissociation constants. Unsaturated acids form all the usual derivatives of acids - salts, anhydrides, acid halides, amides, esters, etc. But due to multiple bonds they enter into addition, oxidation and polymerization reactions.
Due to the mutual influence of the carboxyl group and the multiple bond, the addition of hydrogen halides to a,b-unsaturated acids occurs in such a way that hydrogen is directed to the least hydrogenated carbon atom:
CH 2 = CHCOOH + HBr ® BrCH 2 CH 2 COOH b-bromopropionic acid

Ethylene acids such as acrylic acid and their esters undergo polymerization much more easily than the corresponding hydrocarbons.
individual representatives
Acrylic acid obtained from ethylene (via chlorohydrin or ethylene oxide), by hydrolysis of acrylonitrile or oxidation of propylene, which is more efficient. In technology, derivatives of acrylic acid are used - its esters, especially methyl ( methyl acrylate). Methyl acrylate easily polymerizes to form transparent glassy substances, so it is used in the production of organic glass and other valuable polymers.
Methacrylic acid and its esters are prepared on a large scale by methods similar to those for the synthesis of acrylic acid and its esters. The starting product is acetone, from which acetone cyanohydrin is obtained, subjected to dehydration and saponification to form methacrylic acid. By esterification with methyl alcohol, methyl methacrylate is obtained, which, upon polymerization or copolymerization, forms glassy polymers (organic glasses) with very valuable technical properties.
Dibasic unsaturated acids
The simplest unsaturated dibasic acids are fumaric And maleic - have the same structural formula HOOCCH = CHCOOH, but different spatial configuration: fumaric - trance-, maleic - cis-. Maleic acid (labile form) under the influence of bromine, iodine, nitrous acid easily transforms into a stable (stable) form - fumaric acid. The reverse transition is carried out under the influence of ultraviolet rays. Maleic acid on a technical scale is obtained by the catalytic oxidation of benzene and naphthalene with atmospheric oxygen.
Both acids are capable of forming salts, esters, amides and some other acid derivatives. However, maleic acid, unlike fumaric acid, easily forms a cyclic anhydride, since both carboxyl groups are located on the same side of the double bond ( cis-isomer). Maleic anhydride serves as a characteristic reagent for the detection of 1,3-diene compounds: it readily reacts in diene synthesis and in many cases gives valuable products. Maleic anhydride is widely used in the production of polyester resins and copolymers with styrene, acrylic and methacrylic esters. By hydrating maleic anhydride, malic acid is obtained, which is used in the food industry.
Aromatic monocarboxylic acids
Aromatic carboxylic acids are called benzene derivatives containing carboxyl groups directly bonded to the aromatic ring. Acids containing carboxyl groups in the side chain are considered as fatty-aromatic . Based on the number of carboxylic acid groups, aromatic acids are divided into mono-, dibasic, etc. The name of the acid is derived from the aromatic hydrocarbon (benzoic acid, P-toluic acid).
Methods of obtaining
1. Oxidation of aromatic hydrocarbons .
For the synthesis of aromatic acids, methyl homologues of benzene are most suitable, the radical chain oxidation of which proceeds through the stages of primary hydroperoxide and aldehyde:
ArCH 3 + O 2 ® ArCH2OOH ® ArCHO+ O2 ® ArCOOH
Mono- and dicarboxylic aromatic acids are produced industrially by liquid-phase oxidation of methylbenzenes with atmospheric oxygen.
2. Oxidation of alcohols, aldehydes and ketones .
Aromatic alcohols, aldehydes and ketones oxidize more easily than hydrocarbons. Oxidation is usually carried out using hypochlorites according to the following scheme:
C 6 H 5 - CO - CH 3 + 4 NaOCl ® C 6 H 5 - COOH + NaCl + H 2 O + CO 2
3. Hydrolysis of halogen derivatives .
This method is widely used in technology.
C 6 H 5 CCl 3 + 2 H 2 O ® C 6 H 5 COOH + 3 HCl
When toluene is chlorinated, three types of halogen derivatives are obtained: benzyl chloride for the production of benzyl alcohol; benzylidene chloride - to obtain benzaldehyde; benzotrichloride is converted to benzoic acid.
4. Synthesis Grignard .
C6H5Li + CO2 ® C6H5COOLi + LiBr
Chemical properties
In aqueous solutions, monocarboxylic acids exhibit a greater degree of dissociation than aliphatic acids ( Benzoin acid=6.6×10 -5, Vinegar=1.8×10 -5). The high degree of dissociation of benzoic acid is due to the electrophilic nature of the benzene ring:

The acidity of aromatic acids is almost independent of resonance effects.
Aromatic acids undergo all the reactions that are characteristic of fatty acids. Due to the carboxyl group, various acid derivatives are formed: the action of acids on alkalis and carbonates produces salt , ethers- heating a mixture of acid and alcohol in the presence of mineral acid.
If the substituents in ortho-position is not present, then esterification of the carboxyl group occurs as easily as in the case of aliphatic acids. If one of ortho-positions are substituted, then the rate of esterification is greatly reduced, and if both are occupied ortho-position, then esterification does not occur.
Ethers ortho-substituted benzoic acids can be prepared by reacting silver salts with haloalkanes. They are difficult to hydrolyze. This phenomenon is called spatial (steric) difficulties. Groups larger than hydrogen fill the space around the carbon atom of the carboxyl group to such an extent that it makes it difficult to transition to an intermediate state during the formation or saponification of an ester.
Acid chlorides obtained by acting on acids with thionyl chloride or phosphorus pentachloride:
C 6 H 5 COOH + SOCl 2 ® C 6 H 5 COCl + HCl + SO 2
Anhydrides obtained by distillation of a mixture of acid and acetic anhydride or the action of acid chlorides on salts:
C 6 H 5 COCl + NaOOCC 6 H 5 ® ( C 6 H 5 CO ) 2 O + 2 NaCl
When a salt of an aromatic carboxylic acid is fused with an alkali, the carboxyl group is replaced by hydrogen:
C 6 H 5 COONa + NaOH ® ArH + Na 2 CO 3
The most important representatives
1. Benzoic acid . The main methods for producing benzoic acid are the oxidation of toluene and the decarboxylation of phthalic acid. It is used as a preservative in the food industry due to its strong antiseptic effect, as well as in the production of dyes and fragrances. A very important derivative of benzoic acid is its acid chloride - benzoyl chloride. It is a liquid with a characteristic odor and a strong lachrymatory effect.
2. n-tert -Butylbenzoic acid obtained on an industrial scale by oxidation rubs-butyltoluene in the presence of a soluble cobalt salt as a catalyst. Used in the production of polyester resins.
Dicarboxylic aromatic acids
There are three known benzenedicarboxylic acids: phthalic (O-isomer), isophthalic (m-isomer) and terephthalic (P-isomer). Terephthalic acid is a crystalline substance ( T sublime. 300 o C), compared to isomeric acids, it is least soluble in water and organic liquids. Terephthalic acid and its dimethyl ester play an important role in the production of synthetic fiber lavsan (terylene) - the product of their polycondensation with ethylene glycol. Terephthalic acid is obtained by oxidation P-xylene.
Isophthalic acid is used for the production of polyesters. It is obtained similarly to terephthalic acid - by liquid-phase oxidation m-xylene.
acids - mesotartaric acid is not an optically active substance. The homologue of oxalic acid is adipic acid HOOC(CH 2) 4 COOH, which is obtained by the oxidation of certain cyclic compounds. It is part of cleaning products for removing rust, and also serves as a starting material for the production of polyamide fibers (see the article “Giants of the organic world. Polymers”).
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
Although the carboxyl group consists of carbonyl and hydroxyl groups, carboxylic acids have very different properties from both alcohols and carbonyl compounds. The mutual influence of OH- and -groups leads
to the redistribution of electron density. As a result, the hydrogen atom of the hydroxyl group acquires acidic properties, i.e., it is easily split off when the acid is dissolved in water. Carboxylic acids change the color of indicators and exhibit all the properties characteristic of solutions of inorganic acids.
All monobasic acids that do not contain substituents (for example, formic and acetic acids) are weak - only slightly dissociated into ions. The strength of the acid can be changed by introducing a halogen atom at the a-position to the functional group. Thus, trichloroacetic acid, formed during the chlorination of acetic acid CH 3 COOH + 3Cl 2 ®CCl 3 COOH + 3HCl, in an aqueous solution largely dissociates into ions.
Carboxylic acids can form functional derivatives, the hydrolysis of which again produces the original acids. Thus, when carboxylic acids are exposed to phosphorus(V) chloride and oxide, acid chlorides and anhydrides are formed, respectively; under the action of ammonia and amines - amides; alcohols - esters.
Crystals of monochloroacetic acid CH 2 ClCOOH.
Graph of the dependence of the boiling point of alkanes, alcohols, aldehydes and straight-chain carboxylic acids on the number of carbon atoms in the molecule.
The reaction to form esters is called esterification(from Greek"ether" - "ether"). It is usually carried out in the presence of a mineral acid, which plays the role of a catalyst. When heated, the ester (or water, if the ether boils at a temperature above 100 ° C) is distilled off from the reaction mixture, and the equilibrium shifts to the right. So, from acetic acid and ethyl alcohol, ethyl acetate is obtained - a solvent that is part of many types of glue:
Many esters are colorless liquids with a pleasant odor. Thus, isoamyl acetate smells like pear, ethyl butyrate smells like pineapple, isoamyl butyrate smells like apricot, benzyl acetate smells like jasmine, and ethyl formate smells like rum. Many esters are used as
flavoring additives in the manufacture of various drinks, as well as in perfumery. Derivatives of 2-phenylethyl alcohol have a particularly delicate odor: the ester of this alcohol and phenylacetic acid smells like honey and hyacinths. And the aroma of formic acid ester makes you remember the fragrance of a bouquet of roses and chrysanthemums. In the presence of alkali, esters can be hydrolyzed - decomposed into the original alcohol and a carboxylic acid salt. The hydrolysis of fats (esters of glycerol and higher carboxylic acids) produces the main components of soap - palmitate and sodium stearate,
NAMES OF SOME CARBOXYLIC ACIDS AND THEIR SALTS
*Ethyl acetate is a colorless, water-insoluble liquid with a pleasant ethereal odor ( t kip =77.1 °C), miscible with ethyl alcohol and other organic solvents.
**The names of esters are derived from the names of the corresponding alcohols and acids: ethyl acetate is an ester of ethyl alcohol and acetic acid (ethyl acetyl ester), isoamyl formate is an ester of isoamyl alcohol and formic acid (formic isoamyl ester).
GLACIC ACID
Vinegar, which is formed when wine sours, contains about 5% acetic acid (table vinegar is called a 3-15% solution). By distilling such vinegar, vinegar essence is obtained - a solution with a concentration of 70-80%. And pure (100 percent) acetic acid is released as a result of the action of concentrated sulfuric acid on acetates: CH 3 COOHNa + H 2 SO 4 (conc.) = CH 3 COOH + NaHSO 4.
Such pure acetic acid, which does not contain water, turns into transparent crystals resembling ice when cooled to 16.8 ° C. That's why it is sometimes called icy.
The similarity is not only external: in the crystals there are molecules of acetic acid,
Liquid at room temperature, glacial acetic acid, when cooled below 1–7 °C, turns into colorless crystals that really look like ice.
like water molecules, they form a system of hydrogen bonds. The intermolecular interaction turns out to be so strong that even acetic acid vapor contains not individual molecules, but their agglomerates.
Many salts of acetic acid are unstable to heat. Thus, the decomposition of calcium acetate produces acetone:
And when a mixture of sodium acetate and alkali is heated, methane is released:
For many centuries, the main method for the synthesis of acetic acid was fermentation. Edible vinegar is still produced this way. And for the production of esters and artificial fibers, acid is used as a raw material, which is obtained by the catalytic oxidation of hydrocarbons, for example butane:
CH 3 -CH 2 -CH 2 -CH 3 +2.5O 2 ®2CH 3 -COOH+H 2 O.
Voltaren (ortofen, diclofenac sodium) can be considered the best of modern NSAIDs. It combines a pronounced anti-inflammatory effect with particularly good tolerability, which makes long-term use of the drug possible.
It is absorbed almost completely in the digestive tract, the maximum concentration is reached after 1-2 hours. The drug is actively metabolized and is excreted in the urine and bile in the form of associated metabolic products (some of which also have anti-inflammatory properties). Plasma concentrations are proportional to the dose used. The drug accumulates in areas of inflammation, in particular in the synovial fluid during arthritis, where, unlike plasma, it remains for a long time (up to 7 hours) in an almost unchanged concentration (the concentration in the blood decreases significantly during this period). When prescribed to nursing women, it is practically undetectable in milk. With the simultaneous administration of acetylsalicylic acid and voltaren, the maximum concentration of the latter in plasma is reduced by approximately 30% compared to the administration of voltaren alone.
The inhibitory effect of voltaren on inflammation is apparently based on the active inhibition of prostaglandin synthesis. The drug is the most powerful prostaglandin synthetase inhibitor among modern NSAIDs. Since this inhibition is irreversible [Key E. et al., 1974], its anti-inflammatory effect lasts much longer than the high concentration of the drug in the body. Voltaren is also able to inhibit the action of a number of enzymes involved in the development of the inflammatory process, including lysosomal hydrolases. There is evidence of inhibition of neutral protease isolated from human granulocytes.
The peculiarity of the effect of voltaren can be considered to be such a quickly manifested analgesic effect that even assumptions are made about its partial independence from the anti-inflammatory effect itself. An important feature of the drug was established when studying the dynamics of spontaneous gonarthrosis in mice. It turned out that it reduces its frequency of development and severity of the process, while other NSAIDs (including indomethacin) aggravate this pathology.
This is possibly due to the fact that Voltaren, unlike other drugs, does not negatively affect cartilage metabolism; in particular, in the experiment it does not inhibit the incorporation of sulfur into cartilage proteoglycans.Voltaren is available in a variety of forms: enteric-coated tablets of 25 and 50 mg, slow-release tablets (Voltaren-retard) of 100 mg, suppositories of 50 and 100 mg, in ampoules for intramuscular administration of 75 mg. The drug is prescribed mainly orally in tablet form. The average therapeutic dose is 150 mg, less often - 100 mg; if necessary, the dose is increased to 200 mg. Maintenance doses can be 75-100 mg. Using the drug in suppositories (in the same doses) gives identical results. If you want to achieve a particularly quick effect during the first period of treatment, use intramuscular injections of Voltaren (alone or in addition to taking it orally or in suppositories) 75 mg 1-2 times a day. The pain decreases noticeably 10-45 minutes after the injection. During the period of maintenance therapy, Voltaren-retard is very convenient, which is used at a dose of 100 mg (i.e., 1 tablet) once a day, the drug gives the same effect as taking regular tablets of 25 mg 4 times a day.
Voltaren is most widely used for rheumatoid arthritis, in which case it can be used continuously for many months and years. In mild cases, significant improvement occurs with the use of this drug alone. In seriously ill patients, in accordance with the general principles of treatment of rheumatoid arthritis, treatment with voltaren can be successfully combined with any of the long-acting (basic) drugs. Very good results were also obtained in the treatment of patients with arthrosis. In patients with ankylosing spondylitis, voltaren turned out to be as effective as indomethacin, which was previously considered the best drug, and in terms of tolerability, the advantage of voltaren is undeniable. In fairly high doses (150-200 mg/day), the drug is used to relieve an acute attack of gout; in this case, its intramuscular administration is especially justified.
Recently, it has been established that in the treatment of acute rheumatism, Voltaren, like indomethacin, can have a therapeutic effect similar to that of prednisolone. This applies to all manifestations of the disease, including rheumatic carditis. It is important that the long-term results of prescribing these three drugs were the same. It was found, in particular, that during treatment with voltaren, complete reverse development of valvulitis can occur. The obvious therapeutic effect of voltaren was also noted in other variants of the course of rheumatism, including in a number of patients resistant to other drugs.Good results have been obtained in patients with so-called soft tissue rheumatism (scapulohumeral periarthritis, bursitis, tendonitis, tenosynovitis), as well as in radicular syndromes, including those with acute pain. In the latter cases, injections of the drug are indicated.
The drug is also successfully used for non-rheumatic diseases manifested by inflammation, pain and fever, in particular in patients with thrombophlebitis, adnexitis, infections (in combination with adequate anti-infective agents), in the postoperative period, with bruises, etc. The therapeutic effect is of great importance voltaren for chronic glomerulonephritis, established by G. Lagrue and G. Hirbe (1979), M. Sasdelli et al. (1980). These researchers believe that the drug improves the prognosis of the disease by slowing the rate of progression of kidney failure.
Voltaren is superior to all other NSAIDs in its tolerability. It essentially does not cause severe complications and, if necessary, is used almost constantly. The drug can be used with caution even for peptic ulcers, although, of course, it is advisable to prescribe it in suppositories.
Among the very rare side effects, one should keep in mind mild headache, nausea, abdominal pain, urticaria, the appearance of red blood cells in the urine (apparently due to the weak anticoagulant effect characteristic of all NSAIDs). After reducing the dose or discontinuing the drug, these phenomena quickly disappear. There are no absolute contraindications to the use of Voltaren; Peptic ulcer of the stomach and duodenum in the acute stage may be considered a relative contraindication for oral administration.Tolmetin (tolectin) is a fairly popular anti-rheumatic drug, which is 1-methyl-5p-toluoylpyrrole-2-acetic acid. In some details of the structural formula it resembles indomethacin. Tolmetin is completely absorbed in the digestive tract, the maximum concentration in the blood is observed after 30-40 minutes, the plasma half-purification period lasts about an hour. It is quickly excreted in the urine in the form of glucuronides and inactive metabolites. The mechanism of therapeutic action has not been sufficiently studied; the main importance is given to the inhibition of prostaglandin synthesis.
Available in 200 mg tablets. There are reports of a clear positive effect in patients with rheumatoid arthritis, ankylosing spondylitis, osteoarthritis, glenohumeral periarthritis, epicondylitis, etc. The possibility of long-term use of the drug has been proven, in particular for rheumatoid arthritis up to 2 ½ years. Good results were obtained in the treatment of patients with juvenile rheumatoid arthritis. As is known, new non-steroidal drugs are rarely studied in this disease. It turned out that in terms of the results achieved, tolmetin is not inferior to acetylsalicylic acid, which is still considered the standard anti-inflammatory drug in the treatment of juvenile rheumatoid arthritis. However, despite the unanimously positive overall assessment of tolmetin, this drug belongs to drugs whose analgesic properties prevail over anti-inflammatory ones.
The drug is well tolerated both for short-term and long-term use. Uncommon side effects include epigastric pain, nausea, vomiting, headache, dizziness, tinnitus, skin rashes, fluid retention, and increased blood pressure.
The daily dose is 800-1600 mg (usually about 1200 mg). The drug is often taken 4 times a day due to its rapid elimination from the body.
Functional derivatives of carboxylic acids. Dibasic carboxylic acids. a,b-Unsaturated acids
Carboxylic acid derivatives
1. Acid halides.
When exposed to phosphorus halides or thionyl chloride, the formation of halides occurs:
CH 3 COOH + PCl 5 ® CH 3 COCl + POCl 3 + HCl
The halogen in acid halides is highly reactive. A strong inductive effect determines the ease of substitution of halogen with other nucleophiles: -OH, -OR, -NH2, -N3, -CN, etc.:
CH 3 COCl + CH 3 COOAg ® (CH 3 CO) 2 O acetic anhydride + AgCl
1. Anhydrides.
Anhydrides are formed by the reaction of acid salts with their acid halides:
CH 3 COONa + CH 3 COCl ® NaCl + (CH 3 CO) 2 O
Acid anhydrides are highly chemically active and, like acid halides, are good acylating agents.
Amides are obtained via acid halides
CH 3 COCl +2 NH 3 ® CH 3 CONH 2 acetamide + NH 4 Cl
or from ammonium salts of acids, during dry distillation of which water is split off and an acid amide is formed. Also, acid amides are formed as a by-product during the hydrolysis of nitriles. Amidation processes are important in industry for the production of a number of valuable compounds (N,N-dimethylformamide, dimethylacetamide, ethanolamides of higher acids).
4. Nitriles. The most important representatives of nitriles are acetonitrile CH 3 CN (used as a polar solvent) and acrylonitrile CH 2 =CHCN (monomer for the production of synthetic neuron fiber and for the production of divinylnitrile synthetic rubber, which is oil- and gasoline-resistant). The main method for producing nitriles is the dehydration of amides on acid catalysts:
CH 3 CONH 2 ® CH 3 C-CN + H 2 O
5. Esters. Esters of carboxylic acids are of important practical importance as solvents, hydraulic fluids, lubricating oils, plasticizers and monomers. They are obtained by esterification of alcohols with acids, anhydrides and acid halides or by the reaction of acids and alkenes:
CH 3 -CH=CH 2 + CH 3 COOH ® CH 3 COOCH(CH 3) 2
Many esters are used as aromatic substances:
| CH 3 COOCH 2 CH 3 | pear essence |
| CH 3 CH 2 CH 2 COOCH 2 CH 2 CH 2 CH 2 CH 3 | pineapple essence |
| rum essence |
Dibasic saturated acids
Dibasic saturated (saturated) acids have the general formula C n H 2 n (COOH) 2. Of these, the most important are:
HOOC-COOH - oxalic, ethanedicarboxylic acid;
HOOS-CH 2 -COOH - malonic, propanedicarboxylic acid;
NOOS-CH 2 -CH 2 -COOH - succinic, butanedicarboxylic acid;
NOOS-CH 2 -CH 2 -CH 2 -COOH - glutaric, pentanedicarboxylic acid.
Methods of obtaining
General methods for producing dibasic acids are similar to methods for producing monobasic acids (oxidation of glycols, hydrolysis of dinitriles, Kolbe synthesis - see Lecture No. 27).
1. Oxidation of hydroxy acids:
OH-CH 2 CH 2 COOH ® HOCCH 2 COOH ® HOOC-CH 2 -COOH
2. Oxidation of cycloalkanes.
This is an industrial method for producing adipic acid HOOC-CH 2 CH 2 CH 2 CH 2 -COOH from cyclohexane.
Succinic and oxalic acids are also formed as by-products. Adipic acid is used to synthesize nylon 6,6 fibers and plasticizers.
Chemical properties
Dibasic acids are stronger than monobasic acids. This is explained by the mutual influence of carboxyl groups that facilitate dissociation:

In general, the reactions of dicarboxylic acids and their monocarboxylic analogues are almost the same. The reaction mechanism for the formation of diamides, diesters, etc. from carboxylic acids is the same as for monocarboxylic acids. The exception is dicarboxylic acids, which contain fewer than four carbon atoms between the carboxyl groups. Such acids, whose two carboxyl groups are capable of reacting with the same functional group or with each other, exhibit unusual behavior in reactions that proceed to form five- or six-membered closed activated complexes or products.
An example of the unusual behavior of carboxylic acids is the reactions that occur when heated.
At 150 o C, oxalic acid decomposes into formic acid and CO 2:
HOOC-COOH ® HCOOH + CO 2
2. Cyclodehydration.
When g-dicarboxylic acids, in which the carboxyl groups are separated by carbon atoms, are heated, cyclodehydration occurs, resulting in the formation of cyclic anhydrides: