10. Conditions of chemical equilibrium.
Chemical condition equilibrium- this is a state in which the chemical potential of products and initial substances are equal to each other, taking into account the stoichiometry of the process.
Chemical equilibrium can be talked about when two conditions are met:
The forward and backward reaction rates are equal to each other.
Equilibrium exists if, when an external influence is exerted, and then when it is removed, the system returns to its original state.
11. The law of action of the masses.
At a constant temperature, the rate of a chemical reaction is directly proportional to the product of the concentrations of the reactants taken in powers equal to the stoichiometric coefficients in the reaction equation.
For example, for the ammonia synthesis reaction:
N 2 + 3H 2 = 2NH 3
the law of mass action has the form:
K c = 2 / 3
12. Equilibrium constant in a homogeneous system. Ways of expressing the equilibrium constant.
equilibrium constant Is a constant value equal to the ratio of the products of the equilibrium concentrations of the final and initial participants in the reaction, taken in powers corresponding to the stoichiometric coefficients
Homogeneous are called reactions that take place in one phase: in a mixture of gases, in a liquid or sometimes in a solid solution.
Ways to Express Equilibrium Constants
If the concentrations of the substances participating in the reaction are expressed in molar units of molarity, i.e. in mol / l, then it is usually denoted by Ks
For a homogeneous gas reaction, it is more convenient to express the equilibrium constant in terms of the partial pressures of substances:
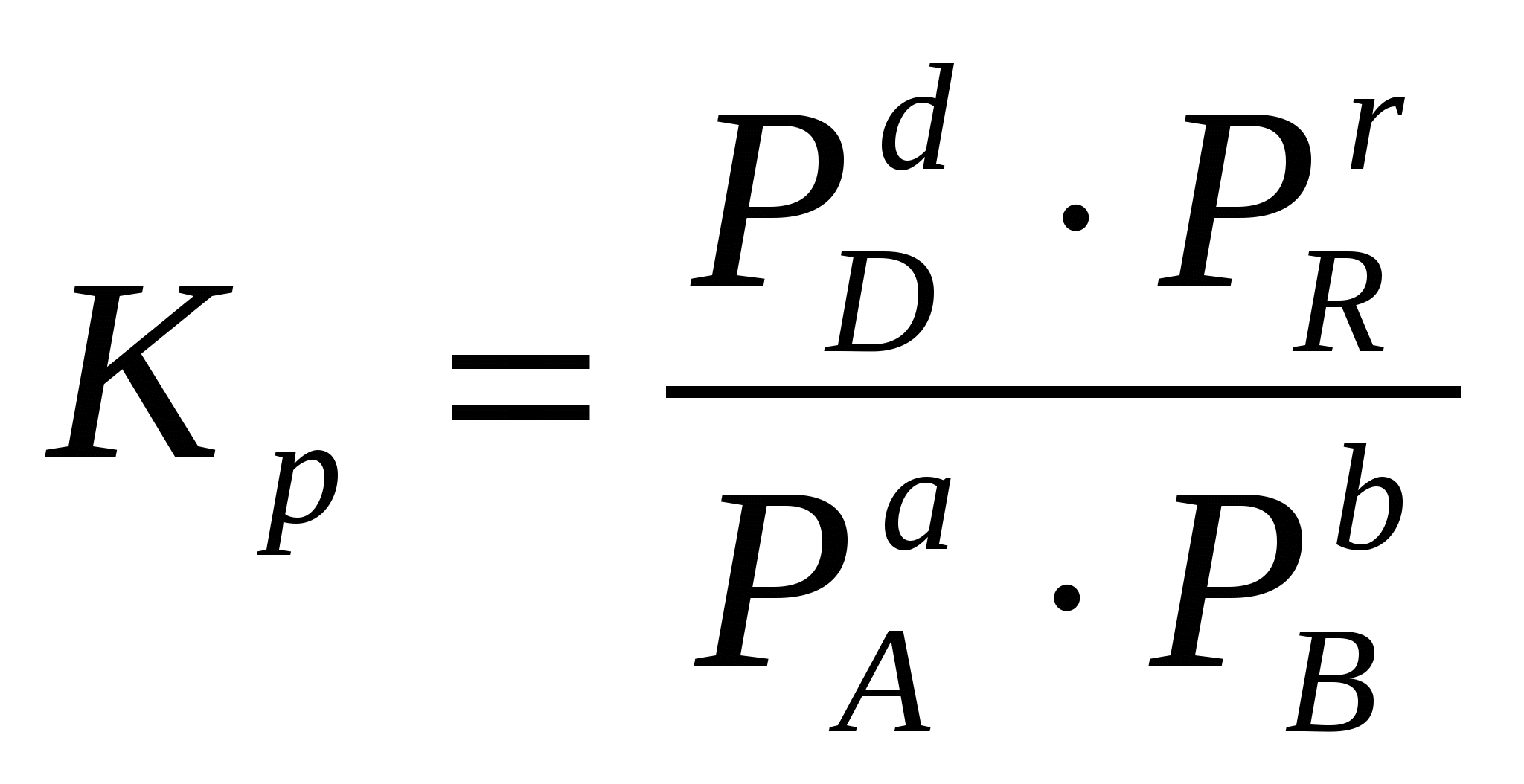
Sometimes it is convenient to express the equilibrium constant not in terms of partial pressures and concentrations, but in terms of the amount of substances: 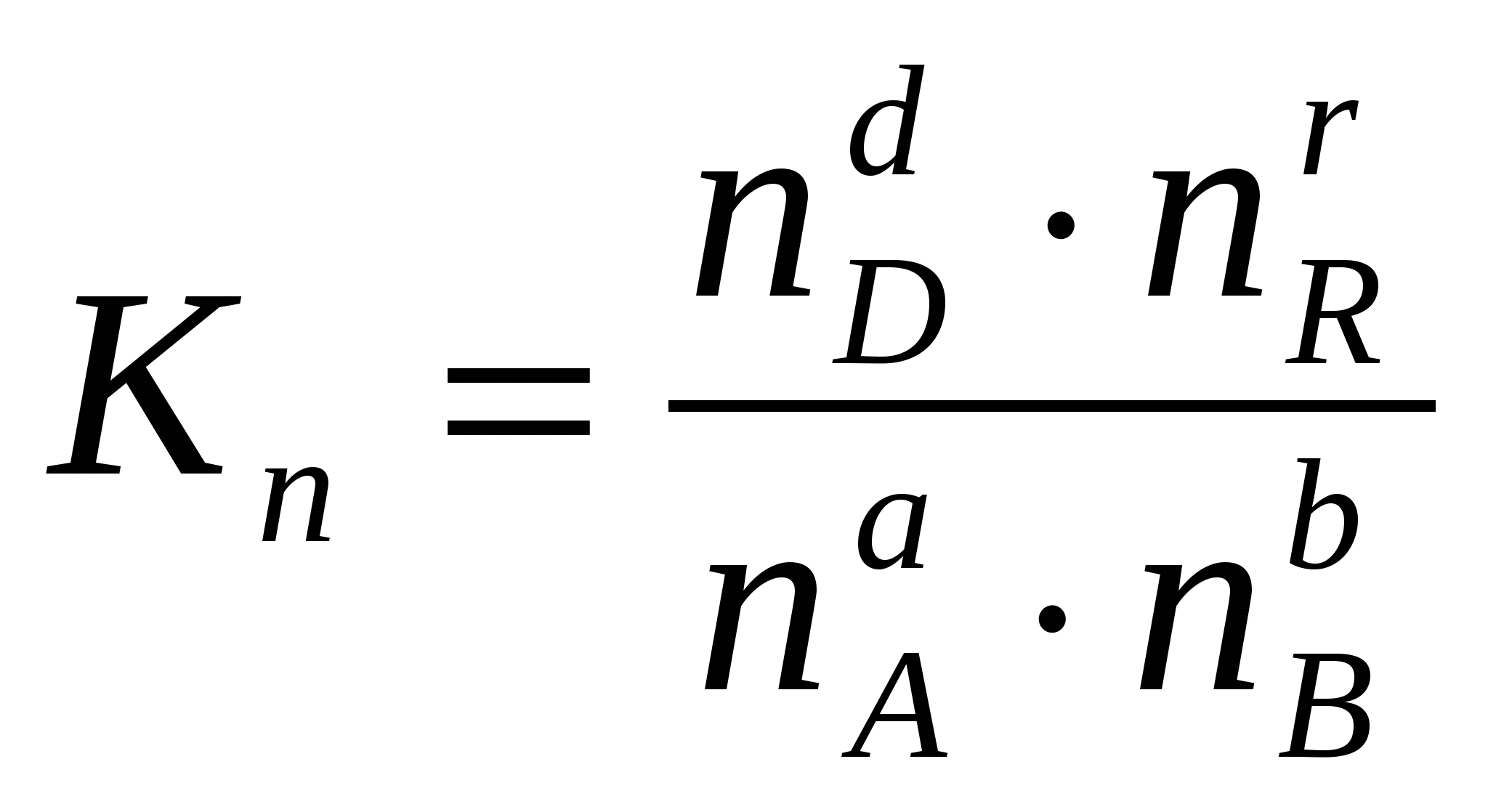 or through the corresponding mole fractions:
or through the corresponding mole fractions: 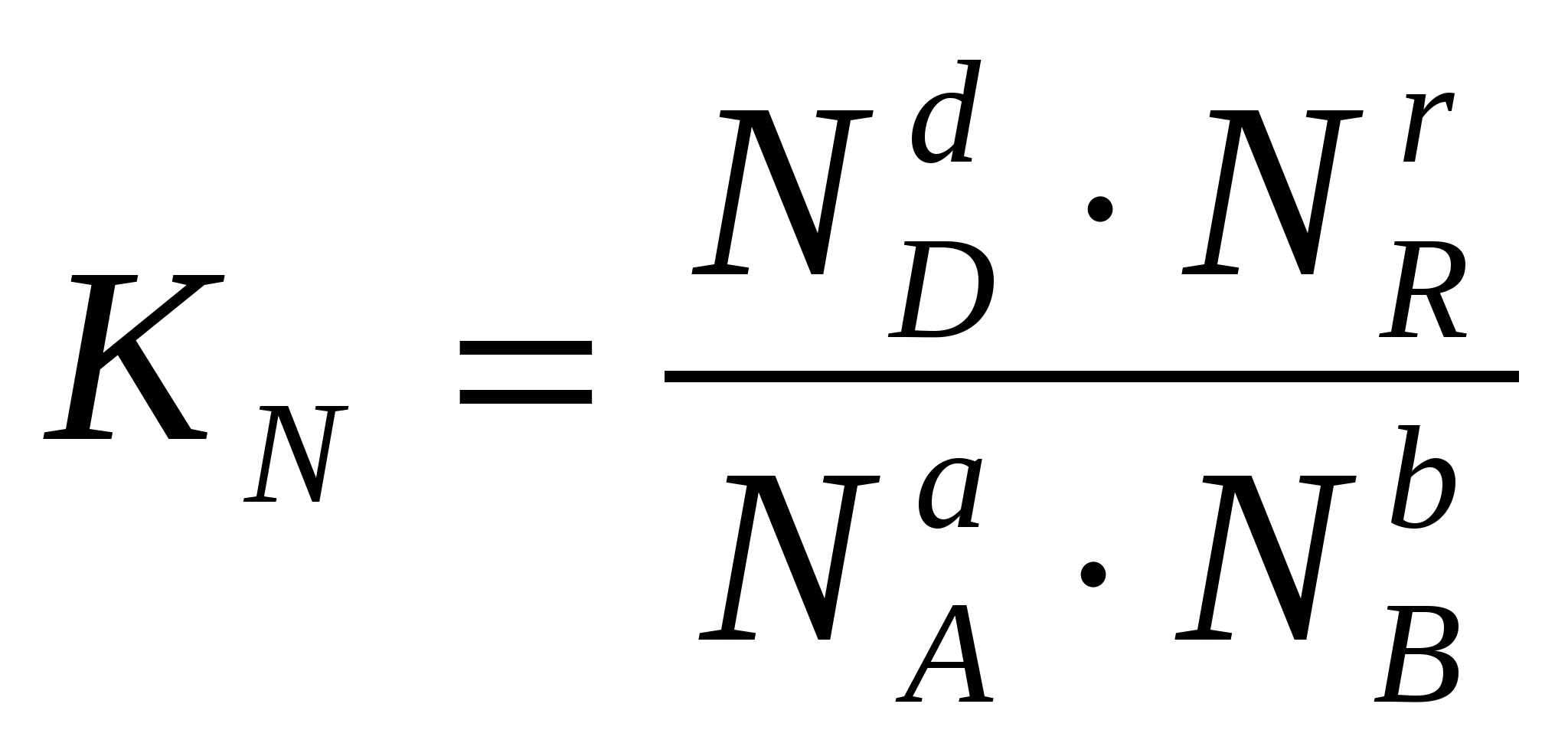
In the general case, the equilibrium constants Kc, Kp, Kn and K N are different.
13 Le Chatelier-Brown Principle .
if an external influence is exerted on a system in equilibrium, then the equilibrium is shifted in the direction that weakens the external influence.
14. Van't Hoff isobar equation.
This expression allows you to qualitatively evaluate the influence of T on equilibrium and the equilibrium constant.
15.Phase.
The phase is called - a homogeneous part of a heterogeneous system, which has a real interface, within which all properties can change continuously, and upon passing through which, in a jump.
16. Ingredients and components.
The component is called- the minimum number of components in-in, sufficient to describe the state of systems.
Constituent substancescalled - substances that make up the system, which can be isolated by conventional drug methods and which can exist outside the system as much as necessary.
17 Gibbs Phase Rule .
The number of degrees of freedom of an equilibrium thermodynamic system, which of external factors is influenced only by temperature and pressure, is equal to the number of independent components C = K-F +n(number of external parameters)
The phase rule shows that the number of degrees of freedom increases with an increase in the number of components and decreases with an increase in the number of phases in the system.
18. Conditions of phase equilibrium in the system.
In a heterogeneous system, there is phase equilibrium if the following types of equilibria exist between the phases:
Thermal (temperature equality)
Mechanical (pressure equality)
Chemical for each component
19.Cliperon-Clausius equation
Where, - Δ V- a change in the volume of a substance during its transition from the first phase to the second, T - transition temperature, Δ H- change in entropy and enthalpy of a substance during the transition of 1 mole of a substance from one phase to another
It allows you to estimate how the temperature or pressure changes during a phase transition with a change in 2 parameters.
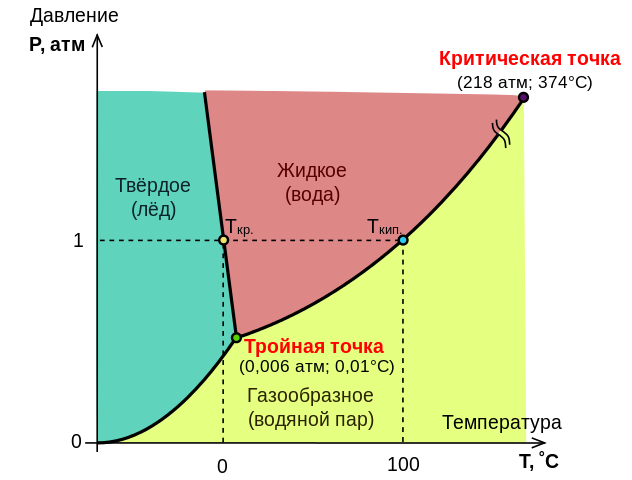
20.Water Condition Diagram
The relationship between the quantities characterizing the state of the system and the phase transformations in the system, the transition from a solid state to a liquid, from a liquid to a gaseous state
21. True solutions. Methods for expressing the concentration of a solution. Calculation of the molar and mass fraction of a substance and the molar concentration of a substance in a solution.
True solution is a type of solution in which the size of the solute particles is extremely small and comparable to the size of the solvent particles.
Solutions are gaseous(gas mixtures), liquid and solid... The gaseous solution is air. Sea water - a mixture of salts in water - a liquid solution. Solid solutions - metal alloys. Solutions are composed of a solvent and a solute (s).
The solution is called a solid or liquid homogeneous system consisting of two or more constituent parts.
The solvent is considered- in-in, which determines the state of aggregation of the solution or in-in, which is greater in volume or mass.
Methods for expressing the concentration of solutions.
Solution concentration Is the mass or amount of a solute in a certain amount, mass or volume of a solution or solvent.
1) Mass fraction ( wi ) Is the mass of the solute contained in 100 grams of solution.
2) Molar fraction (molar) - X i - number of moles of the component contained in 1 mole of solution.
3) Molar concentration (molality) mi - the number of moles of the solute contained in 1 kg of solvent [mol / kg].
4) Molar concentration WITH i - the number of moles of the solute contained in 1 liter or 1 dm3 of the solution [mol / l].