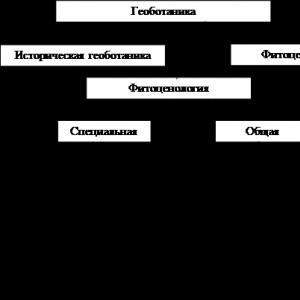
The chemical properties of a simple substance are determined. General characteristics of metals
The chemical properties of a substance depend not only on what chemical elements it consists of, but also on the structure of the molecules of the substance (structural isomerism) and on the spatial configuration of molecules (conformation, stereoisomerism). As a rule, substances with the same composition and structure have the same chemical properties, with the exception of reactions with substances of a different spatial configuration. This difference is especially important in biochemistry, for example, the ability of a protein to react with other biologically active substances may depend on the way it is folded.
Examples of chemical properties
see also
Notes (edit)
Wikimedia Foundation. 2010.
See what "Chemical Properties" is in other dictionaries:
Chemical properties- - determine the material's ability to undergo chemical transformations upon contact with substances of the external environment (including aggressive ones), to preserve the composition and structure in an inert environment, chemical interaction of components ... ...
Chemical properties- - EN chemical property Properties of a substance depending on the arrangement of the atoms in the molecule, e.g. bio availability, degradability, persistence, etc. (Source: RRDA) ... ...
Chemical properties- - a set of electromagnetic interactions between chemical elements, leading to the formation of equilibrium stable systems (molecules, ions, radicals). Analytical Chemistry Dictionary ... Chemical terms
Chemical properties- cheminės savybės statusas T sritis automatika atitikmenys: angl. chemical properties vok. chemische Eigenschaften, f rus. chemical properties, n pranc. propriétés chimiques, f ... Automatikos terminų žodynas
The chemical properties of alcohols are the chemical reactions of alcohols in interaction with other substances. They are mainly determined by the presence of a hydroxyl group and the structure of the hydrocarbon chain, as well as their mutual influence: The more ... ... Wikipedia
Physicochemical characteristics- - characterize the influence of the physical state of the material on the course of certain chemical processes (for example, the degree of dispersion of the material affects the kinetics of chemical reactions). [Kosykh, A. V. Artificial and natural construction ... ... Encyclopedia of terms, definitions and explanations of building materials
Physicochemical properties of refractory raw materials- [refractory] - a set of chemical and / or grain size composition of refractory raw materials [refractory], its thermomechanical and thermophysical properties that determine the field of application. [GOST R 52918 2008] Term heading: Raw materials Encyclopedia headings ... Encyclopedia of terms, definitions and explanations of building materials
The relevance of the subject matter of the article is questioned. Please show in the article the significance of its subject, adding to it evidence of significance by particular criteria of significance or, if particular criteria of significance for ... ... Wikipedia
physical and chemical properties- fizikinės ir cheminės savybės statusas T sritis automatika atitikmenys: angl. physicochemical properties vok. physikalish chemische Eigenschaften, f rus. physical and chemical properties, n pranc. propriétés physico chimiques, f ... Automatikos terminų žodynas
physicochemical characteristics- - [A.S. Goldberg. The English Russian Energy Dictionary. 2006] Topics energy in general EN physicochemical properties ... Technical translator's guide
Books
- Physicochemical properties of semiconductor substances. Directory,. The reference book systematizes the basic properties of pure inorganic crystalline, as well as some glassy substances of elementary, double, triple and more complex ...
All chemical elements are divided into metals and non-metals depending on the structure and properties of their atoms. Also, simple substances formed by elements are classified into metals and non-metals, based on their physical and chemical properties.
In the Periodic Table of Chemical Elements D.I. Mendeleev's non-metals are located diagonally: boron - astatine and above it in the main subgroups.
Comparatively large radii and a small number of electrons at the outer level from 1 to 3 are characteristic of metal atoms (exception: germanium, tin, lead - 4; antimony and bismuth - 5; polonium - 6 electrons).
Nonmetal atoms, on the contrary, are characterized by small atomic radii and the number of electrons at the outer level from 4 to 8 (with the exception of boron, it has three such electrons).
Hence the tendency of metal atoms to give up external electrons, i.e. reducing properties, and for nonmetal atoms - the desire to receive electrons missing to a stable eight-electron level, i.e. oxidizing properties.
Metals
In metals, there is a metallic bond and a metallic crystal lattice. At the lattice sites there are positively charged metal ions, bound by means of socialized external electrons belonging to the entire crystal.
This determines all the most important physical properties of metals: metallic luster, electrical and thermal conductivity, plasticity (the ability to change shape under external influence) and some others characteristic of this class of simple substances.
Metals of group I of the main subgroup are called alkali metals.
Group II metals: calcium, strontium, barium - alkaline earth.
Chemical properties of metals
In chemical reactions, metals exhibit only reducing properties, i.e. their atoms donate electrons, resulting in positive ions.
1. Interact with non-metals:
a) oxygen (with the formation of oxides)
Alkali and alkaline earth metals oxidize easily under normal conditions, so they are stored under a layer of petroleum jelly or kerosene.
4Li + O 2 = 2Li 2 O
2Ca + O 2 = 2CaO
Please note: when sodium interacts - peroxide is formed, potassium - superoxide
2Na + O 2 = Na 2 O 2, K + O2 = KO2
and the oxides are obtained by calcining the peroxide with the corresponding metal:
2Na + Na 2 O 2 = 2Na 2 O
Iron, zinc, copper and other less active metals oxidize slowly in air and actively when heated.
3Fe + 2O 2 = Fe 3 O 4 (a mixture of two oxides: FeO and Fe 2 O 3)
2Zn + O 2 = 2ZnO
2Cu + O 2 = 2CuO
Gold and platinum metals are not oxidized by atmospheric oxygen under any conditions.
b) hydrogen (with the formation of hydrides)
2Na + H 2 = 2NaH
Ca + H 2 = CaH 2
c) chlorine (with the formation of chlorides)
2K + Cl 2 = 2KCl
Mg + Cl 2 = MgCl 2
2Al + 3Cl 2 = 2AlCl 3
Please note: when iron interacts, iron (III) chloride is formed:
2Fe + 3Cl 2 = 2FeCl 3
d) sulfur (with the formation of sulfides)
2Na + S = Na 2 S
Hg + S = HgS
2Al + 3S = Al 2 S 3
Please note: when iron interacts, iron (II) sulfide is formed:
Fe + S = FeS
e) nitrogen (with the formation of nitrides)
6K + N 2 = 2K 3 N
3Mg + N 2 = Mg 3 N 2
2Al + N 2 = 2AlN
2. Interact with complex substances:
It must be remembered that according to their reductive ability, metals are arranged in a row, which is called the electrochemical series of voltages or the activity of metals (displacement series of N.N. Beketov):
Li, K, Ba, Ca, Na, Mg, Al, Mn, Zn, Cr, Fe, Co, Ni, Sn, Pb, (H 2), Cu, Hg, Ag, Au, Pt
a) water
Metals located in a row up to magnesium, under normal conditions, displace hydrogen from water, forming soluble bases - alkalis.
2Na + 2H 2 O = 2NaOH + H 2
Ba + H 2 O = Ba (OH) 2 + H 2
Magnesium interacts with water when boiled.
Mg + 2H 2 O = Mg (OH) 2 + H 2
When removing the oxide film, aluminum reacts violently with water.
2Al + 6H 2 O = 2Al (OH) 3 + 3H 2
The rest of the metals in the row up to hydrogen, under certain conditions, can also react with water with the release of hydrogen and the formation of oxides.
3Fe + 4H 2 O = Fe 3 O 4 + 4H 2
b) acid solutions
(Except concentrated sulfuric acid and nitric acid of any concentration. See "Redox reactions" section.)
Please note: do not use insoluble silicic acid to carry out the reactions
Metals ranging from magnesium to hydrogen displace hydrogen from acids.
Mg + 2HCl = MgCl 2 + H 2
Please note: ferrous salts are formed.
Fe + H 2 SO 4 (dil.) = FeSO 4 + H 2
The formation of insoluble salt prevents the reaction from proceeding. For example, lead practically does not react with sulfuric acid solution due to the formation of insoluble lead sulfate on the surface.
Metals ranked next to hydrogen DO NOT displace hydrogen.
c) salt solutions
Metals that rank up to magnesium and actively react with water are not used to carry out such reactions.
For the rest of the metals, the rule is fulfilled:
Each metal displaces from salt solutions other metals located in a row to the right of it, and itself can be displaced by metals located to the left of it.
Cu + HgCl 2 = Hg + CuCl 2
Fe + CuSO 4 = FeSO 4 + Cu
As with acid solutions, the formation of an insoluble salt prevents the reaction from proceeding.
d) alkali solutions
Metals interact, hydroxides of which are amphoteric.
Zn + 2NaOH + 2H 2 O = Na 2 + H 2
2Al + 2KOH + 6H 2 O = 2K + 3H 2
e) with organic substances
Alkali metals with alcohols and phenol.
2C 2 H 5 OH + 2Na = 2C 2 H 5 ONa + H 2
2C 6 H 5 OH + 2Na = 2C 6 H 5 ONa + H 2
Metals participate in reactions with haloalkanes, which are used to obtain lower cycloalkanes and for syntheses, during which the carbon skeleton of the molecule becomes more complex (A. Würz's reaction):
CH 2 Cl-CH 2 -CH 2 Cl + Zn = C 3 H 6 (cyclopropane) + ZnCl 2
2CH 2 Cl + 2Na = C 2 H 6 (ethane) + 2NaCl
Nonmetals
In simple substances, the atoms of non-metals are linked by a covalent non-polar bond. In this case, single (in H 2, F 2, Cl 2, Br 2, I 2 molecules), double (in O 2 molecules), triple (in N 2 molecules) covalent bonds are formed.
The structure of simple substances - non-metals:
1.molecular
Under normal conditions, most of these substances are gases (H 2, N 2, O 2, O 3, F 2, Cl 2) or solids (I 2, P 4, S 8) and only the only bromine (Br 2) is liquid. All these substances have a molecular structure and are therefore volatile. In the solid state, they are fusible due to the weak intermolecular interaction that holds their molecules in the crystal, and are capable of sublimation.
2.atomic
These substances are formed by crystals, in the nodes of which there are atoms: (B n, C n, Si n, Gen, Se n, Te n). Due to the high strength of covalent bonds, they, as a rule, have a high hardness, and any changes associated with the destruction of the covalent bond in their crystals (melting, evaporation) are performed with a large expenditure of energy. Many of these substances have high melting and boiling points, and their volatility is very low.
Many elements - non-metals form several simple substances - allotropic modifications. Allotropy can be associated with a different composition of molecules: oxygen O 2 and ozone O 3 and with different crystal structures: graphite, diamond, carbyne, fullerene are allotropic modifications of carbon. Elements - non-metals with allotropic modifications: carbon, silicon, phosphorus, arsenic, oxygen, sulfur, selenium, tellurium.
Chemical properties of non-metals
Atoms of non-metals are dominated by oxidizing properties, that is, the ability to attach electrons. This ability is characterized by the value of electronegativity. Among non-metals
At, B, Te, H, As, I, Si, P, Se, C, S, Br, Cl, N, O, F
electronegativity increases and oxidizing properties increase.
From this it follows that for simple substances - non-metals, both oxidizing and reducing properties will be characteristic, with the exception of fluorine, the strongest oxidizing agent.
1. Oxidizing properties
a) in reactions with metals (metals are always reducing agents)
2Na + S = Na 2 S (sodium sulfide)
3Mg + N 2 = Mg 3 N 2 (magnesium nitride)
b) in reactions with non-metals located to the left of the given one, that is, with a lower value of electronegativity. For example, in the interaction of phosphorus and sulfur, sulfur will be the oxidizing agent, since phosphorus has a lower electronegativity value:
2P + 5S = P 2 S 5 (phosphorus sulfide V)
Most non-metals will oxidize with hydrogen:
H 2 + S = H 2 S
H 2 + Cl 2 = 2HCl
3H 2 + N 2 = 2NH 3
c) in reactions with some complex substances
Oxidizing agent - oxygen, combustion reactions
CH 4 + 2O 2 = CO 2 + 2H 2 O
2SO 2 + O 2 = 2SO 3
Oxidizing agent - chlorine
2FeCl 2 + Cl 2 = 2FeCl 3
2KI + Cl 2 = 2KCl + I 2
CH 4 + Cl 2 = CH 3 Cl + HCl
Ch 2 = CH 2 + Br 2 = CH 2 Br-CH 2 Br
2. Restorative properties
a) in reactions with fluorine
S + 3F 2 = SF 6
H 2 + F 2 = 2HF
Si + 2F 2 = SiF 4
b) in reactions with oxygen (except for fluorine)
S + O 2 = SO 2
N 2 + O 2 = 2NO
4P + 5O 2 = 2P 2 O 5
C + O 2 = CO 2
c) in reactions with complex substances - oxidizing agents
H 2 + CuO = Cu + H 2 O
6P + 5KClO 3 = 5KCl + 3P 2 O 5
C + 4HNO 3 = CO 2 + 4NO 2 + 2H 2 O
H 2 C = O + H 2 = CH 3 OH
3. Disproportionation reactions: the same non-metal is both an oxidizing agent and a reducing agent
Cl 2 + H 2 O = HCl + HClO
3Cl 2 + 6KOH = 5KCl + KClO 3 + 3H 2 O
If in the periodic table of elements of D.I. Mendeleev we draw a diagonal from beryllium to astatine, then on the left below the diagonal there will be metal elements (these also include elements of side subgroups, highlighted in blue), and on the top right - nonmetal elements (highlighted yellow). Elements located near the diagonal - semimetals or metalloids (B, Si, Ge, Sb, etc.), have a dual character (highlighted in pink).
As you can see from the figure, the vast majority of elements are metals.
By their chemical nature, metals are chemical elements whose atoms donate electrons from an external or pre-external energy level, thus forming positively charged ions.
Almost all metals have relatively large radii and a small number of electrons (from 1 to 3) at the external energy level. Metals are characterized by low values of electronegativity and reducing properties.
The most typical metals are located at the beginning of the periods (starting from the second), further from left to right, the metallic properties weaken. In the group from top to bottom, metallic properties are enhanced, because the radius of the atoms increases (due to an increase in the number of energy levels). This leads to a decrease in the electronegativity (the ability to attract electrons) of elements and an increase in the reducing properties (the ability to donate electrons to other atoms in chemical reactions).
Typical metals are s-elements (elements of the IA-group from Li to Fr. elements of the PA-group from Mg to Ra). The general electronic formula of their atoms is ns 1-2. They are characterized by the oxidation states + I and + II, respectively.
A small number of electrons (1-2) at the outer energy level of typical metal atoms suggests a slight loss of these electrons and the manifestation of strong reducing properties, which reflect low values of electronegativity. Hence, the chemical properties and methods of obtaining typical metals are limited.
A characteristic feature of typical metals is the tendency of their atoms to form cations and ionic chemical bonds with nonmetal atoms. Compounds of typical metals with non-metals are ionic crystals "metal cation anion of non-metal", for example K + Br -, Ca 2+ O 2-. Cations of typical metals are also included in compounds with complex anions - hydroxides and salts, for example, Mg 2+ (OH -) 2, (Li +) 2CO 3 2-.
Metals of A-groups forming a diagonal of amphotericity in the Periodic Table Be-Al-Ge-Sb-Po, as well as adjacent metals (Ga, In, Tl, Sn, Pb, Bi) do not exhibit typically metallic properties. The general electronic formula of their atoms ns 2 np 0-4 suggests a greater variety of oxidation states, a greater ability to hold their own electrons, a gradual decrease in their reductive ability and the appearance of oxidizing ability, especially in high oxidation states (typical examples are compounds Tl III, Pb IV, Bi v). A similar chemical behavior is typical for most (d-elements, i.e., elements of B-groups of the Periodic Table (typical examples are amphoteric elements Cr and Zn).
This manifestation of the duality (amphotericity) of properties, both metallic (basic) and non-metallic, is due to the nature of the chemical bond. In the solid state, compounds of atypical metals with non-metals contain predominantly covalent bonds (but less strong than bonds between non-metals). In solution, these bonds are easily broken, and the compounds dissociate into ions (in whole or in part). For example, gallium metal consists of Ga 2 molecules, in the solid state aluminum and mercury (II) chlorides AlCl 3 and HgCl 2 contain strongly covalent bonds, but in a solution of AlCl 3 it dissociates almost completely, and HgCl 2 - to a very small extent (and then on ions НgСl + and Сl -).
General physical properties of metals
Due to the presence of free electrons ("electron gas") in the crystal lattice, all metals exhibit the following characteristic general properties:
1) Plastic- the ability to easily change shape, be drawn into wire, rolled into thin sheets.
2) Metallic luster and opacity. This is due to the interaction of free electrons with light incident on the metal.
3) Electrical conductivity... It is explained by the directional movement of free electrons from the negative to the positive pole under the influence of a small potential difference. When heated, the electrical conductivity decreases, because with an increase in temperature, the vibrations of atoms and ions in the nodes of the crystal lattice intensify, which complicates the directional movement of the "electron gas".
4) Thermal conductivity. It is caused by the high mobility of free electrons, due to which there is a rapid equalization of temperature over the mass of the metal. Bismuth and mercury have the highest thermal conductivity.
5) Hardness. The hardest is chrome (cuts glass); the softest - alkali metals - potassium, sodium, rubidium and cesium - are cut with a knife.
6) Density. The smaller the atomic mass of the metal and the larger the radius of the atom, the smaller it is. The lightest is lithium (ρ = 0.53 g / cm3); the heaviest is osmium (ρ = 22.6 g / cm3). Metals with a density of less than 5 g / cm3 are considered "light metals".
7) Melting and boiling points. The lowest-melting metal is mercury (melting point = -39 ° C), the most refractory metal is tungsten (melting point = 3390 ° C). Metals with t ° pl. above 1000 ° C are considered refractory, below - low melting.
General chemical properties of metals
Strong reducing agents: Me 0 - nē → Me n +
A number of stresses characterize the comparative activity of metals in redox reactions in aqueous solutions. 
I. Reactions of metals with non-metals
1) With oxygen:
2Mg + O 2 → 2MgO
2) With gray:
Hg + S → HgS
3) With halogens:
Ni + Cl 2 - t ° → NiCl 2
4) With nitrogen:
3Ca + N 2 - t ° → Ca 3 N 2
5) With phosphorus:
3Ca + 2P - t ° → Ca 3 P 2
6) With hydrogen (only alkali and alkaline earth metals react):
2Li + H 2 → 2LiH
Ca + H 2 → CaH 2
II. Reactions of metals with acids
1) Metals in the electrochemical series of voltages up to H reduce non-oxidizing acids to hydrogen:
Mg + 2HCl → MgCl 2 + H 2
2Al + 6HCl → 2AlCl 3 + 3H 2
6Na + 2H 3 PO 4 → 2Na 3 PO 4 + 3H 2
2) With oxidizing acids:
With the interaction of nitric acid of any concentration and concentrated sulfuric with metals hydrogen is never released! 
Zn + 2H 2 SO 4 (К) → ZnSO 4 + SO 2 + 2H 2 O
4Zn + 5H 2 SO 4 (К) → 4ZnSO 4 + H 2 S + 4H 2 O
3Zn + 4H 2 SO 4 (К) → 3ZnSO 4 + S + 4H 2 O
2H 2 SO 4 (k) + Cu → Cu SO 4 + SO 2 + 2H 2 O
10HNO 3 + 4Mg → 4Mg (NO 3) 2 + NH 4 NO 3 + 3H 2 O
4HNO 3 (c) + Cu → Cu (NO 3) 2 + 2NO 2 + 2H 2 O
III. Interaction of metals with water
1) Active (alkali and alkaline earth metals) form a soluble base (alkali) and hydrogen:
2Na + 2H 2 O → 2NaOH + H 2
Ca + 2H 2 O → Ca (OH) 2 + H 2
2) Metals of medium activity are oxidized by water when heated to oxide:
Zn + H 2 O - t ° → ZnO + H 2
3) Inactive (Au, Ag, Pt) - do not react.
IV. Displacement of less active metals from solutions of their salts by more active metals:
Cu + HgCl 2 → Hg + CuCl 2
Fe + CuSO 4 → Cu + FeSO 4

In industry, not pure metals are often used, but their mixtures - alloys, in which the beneficial properties of one metal are complemented by the beneficial properties of another. So, copper has a low hardness and is of little use for the manufacture of machine parts, while copper-zinc alloys ( brass) are already quite solid and are widely used in mechanical engineering. Aluminum has high ductility and sufficient lightness (low density), but too soft. On its basis, an alloy with magnesium, copper and manganese is prepared - duralumin (duralumin), which, without losing the useful properties of aluminum, acquires high hardness and becomes suitable in aircraft construction. Alloys of iron with carbon (and additives of other metals) are widely known cast iron and steel.
Free metals are reducing agents. However, the reactivity of some metals is low due to the fact that they are coated surface oxide film, in varying degrees, resistant to the action of chemicals such as water, solutions of acids and alkalis.
For example, lead is always covered with an oxide film; for its transition into solution, not only the action of a reagent (for example, dilute nitric acid) is required, but also heating. The oxide film on aluminum prevents it from reacting with water, but is destroyed by acids and alkalis. Loose oxide film (rust), formed on the surface of iron in humid air, does not interfere with further oxidation of iron.
Under the influence concentrated acids on metals are formed steady oxide film. This phenomenon is called passivation... So, in concentrated sulfuric acid metals such as Be, Bi, Co, Fe, Mg and Nb are passivated (and then do not react with acid), and metals A1, Be, Bi, Co, Cr, Fe, Nb, Ni, Pb in concentrated nitric acid , Th and U.
When interacting with oxidants in acidic solutions, most metals are converted into cations, the charge of which is determined by the stable oxidation state of a given element in compounds (Na +, Ca 2+, A1 3+, Fe 2+ and Fe 3+)
The reducing activity of metals in an acidic solution is transmitted by a series of voltages. Most of the metals are converted into a solution with hydrochloric and dilute sulfuric acids, but Cu, Ag and Hg - only sulfuric (concentrated) and nitric acids, and Pt and Au - "aqua regia".
Corrosion of metals
An undesirable chemical property of metals is their, i.e., active destruction (oxidation) upon contact with water and under the influence of oxygen dissolved in it (oxygen corrosion). For example, corrosion of iron products in water is widely known, as a result of which rust is formed and the products are crumbled into powder.
Corrosion of metals occurs in water also due to the presence of dissolved gases CO 2 and SO 2; an acidic environment is created, and H + cations are displaced by active metals in the form of hydrogen H 2 ( hydrogen corrosion).
The place of contact of two dissimilar metals ( contact corrosion). A galvanic pair arises between one metal, such as Fe, and another metal, such as Sn or Cu, placed in water. The flow of electrons goes from the more active metal, which is to the left in the series of voltages (Fe), to the less active metal (Sn, Cu), and the more active metal is destroyed (corroded).
It is because of this that the tinned surface of cans (tin-coated iron) rusts when stored in a humid atmosphere and carelessly handling them (iron quickly collapses after the appearance of at least a small scratch that allows iron to come into contact with moisture). On the contrary, the galvanized surface of an iron bucket does not rust for a long time, because even in the presence of scratches, it is not iron that corrodes, but zinc (a more active metal than iron).
Corrosion resistance for a given metal is enhanced when it is coated with a more active metal or when they are fused; thus, plating iron with chromium or making an iron-chromium alloy eliminates the corrosion of iron. Chromium-plated iron and steel containing chromium ( stainless steel), have high corrosion resistance.
electrometallurgy, i.e., obtaining metals by electrolysis of melts (for the most active metals) or salt solutions;
pyrometallurgy, i.e., the recovery of metals from ores at high temperatures (for example, the production of iron in a blast furnace);
hydrometallurgy, i.e., the separation of metals from solutions of their salts with more active metals (for example, obtaining copper from a CuSO 4 solution by the action of zinc, iron or aluminum).
Native metals are sometimes found in nature (typical examples are Ag, Au, Pt, Hg), but more often metals are in the form of compounds ( metal ores). In terms of prevalence in the earth's crust, metals are different: from the most common - Al, Na, Ca, Fe, Mg, K, Ti) to the rarest - Bi, In, Ag, Au, Pt, Re.
Group IIA contains only metals - Be (beryllium), Mg (magnesium), Ca (calcium), Sr (strontium), Ba (barium) and Ra (radium). The chemical properties of the first representative of this group, beryllium, are most different from the chemical properties of the rest of the elements of this group. Its chemical properties are in many ways even more similar to aluminum than to other metals of IIA group (the so-called "diagonal similarity"). Magnesium, on the other hand, differs markedly from Ca, Sr, Ba and Ra in chemical properties, but it still has much more similar chemical properties with them than with beryllium. Due to the significant similarity of the chemical properties of calcium, strontium, barium and radium, they are combined into one family, called alkaline earth metals.
All elements of the IIA group belong to s-elements, i.e. contain all their valence electrons on s-sub-level. Thus, the electronic configuration of the outer electron layer of all chemical elements of a given group has the form ns 2 , where n- number of the period in which the element is located.
Due to the peculiarities of the electronic structure of group IIA metals, these elements, in addition to zero, are capable of having only one single oxidation state equal to +2. Simple substances formed by the elements of group IIA, when participating in any chemical reactions, can only be oxidized, i.e. donate electrons:
Ме 0 - 2e - → Ме +2
Calcium, strontium, barium and radium are extremely reactive. The simple substances formed by them are very strong reducing agents. Magnesium is also a powerful reducing agent. The reducing activity of metals obeys the general laws of the periodic law of D.I. Mendeleev and increases down the subgroup.
Interaction with simple substances
with oxygen
Without heating, beryllium and magnesium do not react either with atmospheric oxygen or with pure oxygen due to the fact that they are covered with thin protective films consisting, respectively, of BeO and MgO oxides. Their storage does not require any special methods of protection from air and moisture, unlike alkaline earth metals, which are stored under a layer of liquid inert to them, most often kerosene.
Be, Mg, Ca, Sr, when burning in oxygen, form oxides of the composition MeO, and Ba - a mixture of barium oxide (BaO) and barium peroxide (BaO 2):
2Mg + O 2 = 2MgO
2Ca + O 2 = 2CaO
2Ba + O 2 = 2BaO
Ba + O 2 = BaO 2
It should be noted that during the combustion of alkaline earth metals and magnesium in air, the reaction of these metals with nitrogen in the air also occurs as a side effect, as a result of which, in addition to compounds of metals with oxygen, nitrides with the general formula Me 3 N 2 are also formed.
with halogens
Beryllium reacts with halogens only at high temperatures, and the rest of the IIA group metals - already at room temperature:
Mg + I 2 = MgI 2 - magnesium iodide
Ca + Br 2 = CaBr 2 - calcium bromide
Ba + Cl 2 = BaCl 2 - barium chloride
with non-metals of IV-VI groups
All metals of group IIA react when heated with all non-metals of IV-VI groups, but depending on the position of the metal in the group, as well as the activity of non-metals, a different degree of heating is required. Since beryllium is the most chemically inert among all IIA group metals, it requires substantially b O higher temperature.
It should be noted that the reaction of metals with carbon can form carbides of different nature. Distinguish between carbides belonging to methanides and conditionally considered derivatives of methane, in which all hydrogen atoms are replaced by metal. They, like methane, contain carbon in the oxidation state -4, and during their hydrolysis or interaction with non-oxidizing acids, one of the products is methane. There is also another type of carbides - acetylenides, which contain the C 2 2- ion, which is actually a fragment of the acetylene molecule. Carbides of the acetylenide type upon hydrolysis or interaction with non-oxidizing acids form acetylene as one of the reaction products. What type of carbide - methanide or acetylenide - is obtained by the interaction of a particular metal with carbon depends on the size of the metal cation. With metal ions with a small radius, methanides are formed, as a rule, with ions of a larger size, acetylenides. In the case of metals of the second group, methanide is obtained by the interaction of beryllium with carbon:
The rest of the II A group metals form acetylenides with carbon:
With silicon, group IIA metals form silicides - compounds of the type Me 2 Si, with nitrogen - nitrides (Me 3 N 2), phosphorus - phosphides (Me 3 P 2):
with hydrogen
All alkaline earth metals react with hydrogen when heated. In order for magnesium to react with hydrogen, heating alone, as is the case with alkaline earth metals, is not enough; in addition to a high temperature, an increased pressure of hydrogen is also required. Beryllium does not react with hydrogen under any circumstances.
Interaction with complex substances
with water
All alkaline earth metals actively react with water to form alkalis (soluble metal hydroxides) and hydrogen. Magnesium reacts with water only when boiling due to the fact that when heated, the protective oxide film of MgO dissolves in water. In the case of beryllium, the protective oxide film is very stable: water does not react with it either during boiling, or even at red heat:
with non-oxidizing acids
All metals of the main subgroup of group II react with non-oxidizing acids, since they are in the line of activity to the left of hydrogen. This forms the salt of the corresponding acid and hydrogen. Examples of reactions:
Be + H 2 SO 4 (dil.) = BeSO 4 + H 2
Mg + 2HBr = MgBr 2 + H 2
Ca + 2CH 3 COOH = (CH 3 COO) 2 Ca + H 2
with oxidizing acids
- diluted nitric acid
All metals of group IIA react with dilute nitric acid. In this case, the reduction products instead of hydrogen (as in the case of non-oxidizing acids) are nitrogen oxides, mainly nitrogen oxide (I) (N 2 O), and in the case of highly dilute nitric acid, ammonium nitrate (NH 4 NO 3):
4Ca + 10HNO 3 ( smashed .) = 4Ca (NO 3) 2 + N 2 O + 5H 2 O
4Mg + 10HNO 3 (badly broken)= 4Mg (NO 3) 2 + NH 4 NO 3 + 3H 2 O
- concentrated nitric acid
Concentrated nitric acid passivates beryllium at ordinary (or low) temperatures, i.e. does not react with it. When boiling, the reaction is possible and proceeds mainly in accordance with the equation:
Magnesium and alkaline earth metals react with concentrated nitric acid to form a wide range of different nitrogen reduction products.
- concentrated sulfuric acid
Beryllium is passivated with concentrated sulfuric acid, i.e. does not react with it under normal conditions, however, the reaction proceeds during boiling and leads to the formation of beryllium sulfate, sulfur dioxide and water:
Be + 2H 2 SO 4 → BeSO 4 + SO 2 + 2H 2 O
Barium is also passivated by concentrated sulfuric acid due to the formation of insoluble barium sulfate, but reacts with it when heated; barium sulfate dissolves when heated in concentrated sulfuric acid due to its conversion to barium hydrogen sulfate.
The rest of the metals of the main IIA group react with concentrated sulfuric acid under any conditions, including in the cold. Sulfur reduction can occur to SO 2, H 2 S and S, depending on the activity of the metal, the reaction temperature and the acid concentration:
Mg + H 2 SO 4 ( end .) = MgSO 4 + SO 2 + H 2 O
3Mg + 4H 2 SO 4 ( end .) = 3MgSO 4 + S ↓ + 4H 2 O
4Ca + 5H 2 SO 4 ( end .) = 4CaSO 4 + H 2 S + 4H 2 O
with alkalis
Magnesium and alkaline earth metals do not interact with alkalis, and beryllium easily reacts both with alkali solutions and with anhydrous alkalis during fusion. In this case, when the reaction is carried out in an aqueous solution, water also participates in the reaction, and the products are tetrahydroxoberyllates of alkali or alkaline earth metals and gaseous hydrogen:
Be + 2KOH + 2H 2 O = H 2 + K 2 - potassium tetrahydroxoberyllate
When the reaction is carried out with a solid alkali during fusion, beryllates of alkali or alkaline earth metals and hydrogen are formed
Be + 2KOH = H 2 + K 2 BeO 2 - potassium beryllate
with oxides
Alkaline earth metals, as well as magnesium, can reduce less active metals and some non-metals from their oxides when heated, for example:
The method of reducing metals from their oxides with magnesium is called magnesiumthermia.
Inorganic substances are simple and complex. Simple substances are divided into metals (K, Na, Li) and non-metals (O, Cl, P). Complex substances are divided into oxides, hydroxides (bases), salts and acids.
Oxides
Oxides- compounds of a chemical element (metal or non-metal) with oxygen (oxidation state -2), while oxygen is associated with a less electronegative element.
Allocate:
1. Acid oxides- oxides showing acidic properties. Formed by non-metals and oxygen. Examples: SO3, SO2, CO2, P2O5, N2O5.
2. Amphoteric oxides- oxides that can exhibit both basic and acidic properties (this property is called amphotericity). Examples: Al2O3, CrO3, ZnO, BeO, PbO.
3. Basic oxides- metal oxides, while the metals exhibit an oxidation state of +1 or +2. Examples: K2O, MgO, CaO, BaO, Li2O, Na2O.
4. Non-salt-forming oxides- practically do not enter into reactions, do not have corresponding acids and hydroxides. Examples: CO, NO.
Chemical properties of basic oxides
1. Interaction with water
Only oxides of alkali and alkaline earth metals enter into the reaction, the hydroxides of which form a soluble base
basic oxide + water → alkali
K2O + H2O → 2KOH
CaO + H2O → Ca (OH) 2
2. Interaction with acid
basic oxide + acid → salt + water
MgO + H2SO4 → MgSO4 + H2O
Na2O + H2S (g) → 2NaHS + H2O
MgO (g) + HCl → Mg (OH) Cl
3. Interaction with acidic or amphoteric oxides
basic oxide + acidic / amphoteric oxide → salt
In this case, the metal in the basic oxide becomes a cation, and the acidic / amphoteric oxide becomes an anion (acidic residue). Reactions between solid oxides occur when heated. Water-insoluble basic oxides do not interact with gaseous acidic oxides.
BaO + SiO2 (t) → BaSiO3
K2O + ZnO (t) → K2ZnO2
FeO + CO2 ≠
4. Interaction with amphoteric hydroxides
basic oxide + amphoteric hydroxide → salt + water
Na2O + 2Al (OH) 3 (t) → 2NaAlO2 + 3H2O
5. Decomposition at a temperature of oxides of noble metals and mercury
2Ag2O (t) → 4Ag + O2
2HgO (t) → 2Hg + O2
6. Interaction with carbon (C) or hydrogen (H2) at high temperatures.
When the oxides of alkali, alkaline earth metals and aluminum are reduced in this way, not the metal itself is released, but its carbide.
FeO + C (t) → Fe + CO
3Fe2O3 + C (t) → 2Fe3O4 + CO
CaO + 3C (t) → CaC2 + CO
CaO + 2H2 (t) → CaH2 + H2O
7. Active metals reduce less active metals from their oxides at high temperatures
CuO + Zn (t) → ZnO + Cu
8. Oxygen oxidizes lower oxides to higher ones.
Alkali and alkaline earth metal oxides are converted to peroxides
4FeO + O2 (t) → 2Fe2O3
2BaO + O2 (t) → 2BaO2
2NaO + O2 (t) → 2Na2O2
Chemical properties of acidic oxides
1. Interaction with water
acid oxide + water → acid
SO3 + H2O → H2SO4
SiO2 + H2O ≠
Some oxides do not have corresponding acids, in which case a disproportionation reaction occurs
2NO2 + H2O → HNO3 + HNO2
3NO2 + H2O (t) → 2HNO3 + NO
2ClO2 + H2O → HClO3 + HClO2
6ClO2 + 3H2O (t) → 5HClO3 + HCl
Depending on the number of water molecules attached to P2O5, three different acids are formed - metaphosphoric НРО3, pyrophosphoric Н4Р2О7 or orthophosphoric Н3РО4.
P2O5 + H2O → 2HPO3
P2O5 + 2H2O → H4P2O7
P2O5 + 3H2O → 2H3PO4
Chromium oxide corresponds to two acids - chromic H2CrO4 and dichromic H2Cr2O7 (III)
CrO3 + H2O → H2CrO4
2CrO3 + H2O → H2Cr2O7
2. Interaction with bases
acid oxide + base → salt + water
Insoluble acidic oxides react only upon fusion, and soluble ones under normal conditions.
SiO2 + 2NaOH (t) → Na2SiO3 + H2O
With an excess of oxide, an acidic salt is formed.
CO2 (g) + NaOH → NaHCO3
P2O5 (g) + 2Ca (OH) 2 → 2CaHPO4 + H2O
P2O5 (g) + Ca (OH) 2 + H2O → Ca (H2PO4) 2
With an excess of base, a basic salt is formed
CO2 + 2Mg (OH) 2 (g) → (MgOH) 2CO3 + H2O
Oxides that do not have corresponding acids undergo disproportionation reactions and form two salts.
2NO2 + 2NaOH → NaNO3 + NaNO2 + H2O
2ClO2 + 2NaOH → NaClO3 + NaClO2 + H2O
CO2 reacts with some amphoteric hydroxides (Be (OH) 2, Zn (OH) 2, Pb (OH) 2, Cu (OH) 2) to form basic salt and water.
CO2 + 2Be (OH) 2 → (BeOH) 2CO3 ↓ + H2O
CO2 + 2Cu (OH) 2 → (CuOH) 2CO3 ↓ + H2O
3. Interaction with basic or amphoteric oxide
acidic oxide + basic / amphoteric oxide → salt
Reactions between solid oxides take place during fusion. Amphoteric and water-insoluble basic oxides interact only with solid and liquid acidic oxides.
SiO2 + BaO (t) → BaSiO3
3SO3 + Al2O3 (t) → Al2 (SO4) 3
4. Interaction with salt
acidic non-volatile oxide + salt (t) → salt + acidic volatile oxide
SiO2 + CaCO3 (t) → CaSiO3 + CO2
P2O5 + Na2CO3 → 2Na3PO4 + 2CO2
5. Acidic oxides do not interact with acids, but P2O5 reacts with anhydrous oxygen-containing acids.
In this case, HPO3 and the corresponding acid anhydride are formed
P2O5 + 2HClO4 (anhydrous) → Cl2O7 + 2HPO3
P2O5 + 2HNO3 (anhydrous) → N2O5 + 2HPO3
6. Enter into redox reactions.
1. Recovery
At high temperatures, some non-metals can reduce oxides.
CO2 + C (t) → 2CO
SO3 + C → SO2 + CO
H2O + C (t) → H2 + CO
Magnesium thermal is often used to reduce non-metals from their oxides.
CO2 + 2Mg → C + 2MgO
SiO2 + 2Mg (t) → Si + 2MgO
N2O + Mg (t) → N2 + MgO
2. Lower oxides are converted into higher ones when interacting with ozone (or oxygen) at high temperatures in the presence of a catalyst
NO + O3 → NO2 + O2
SO2 + O3 → SO3 + O2
2NO2 + O3 → N2O5 + O2
2CO + O2 (t) → 2CO2
2SO2 + O2 (t, kat) → 2SO3
P2O3 + O2 (t) → P2O5
2NO + O2 (t) → 2NO2
2N2O3 + O2 (t) → 2N2O4
3. Oxides also enter into other redox reactions
SO2 + NO2 → NO + SO3 4NO2 + O2 + 2H2O → 4HNO3
2SO2 + 2NO → N2 + 2SO3 2N2O5 → 4NO2 + O2
SO2 + 2H2S → 3S ↓ + 2H2O 2NO2 (t) → 2NO + O2
2SO2 + O2 + 2H2O → 2H2SO4 3N2O + 2NH3 → 4N2 + 3H2O
2CO2 + 2Na2O2 → 2Na2CO3 + O2 10NO2 + 8P → 5N2 + 4P2O5
N2O + 2Cu (t) → N2 + Cu2O
2NO + 4Cu (t) → N2 + 2Cu2O
N2O3 + 3Cu (t) → N2 + 3CuO
2NO2 + 4Cu (t) → N2 + 4CuO
N2O5 + 5Cu (t) → N2 + 5CuO
Chemical properties of amphoteric oxides
1. Do not interact with water
amphoteric oxide + water ≠
2. Interaction with acids
amphoteric oxide + acid → salt + water
Al2O3 + 3H2SO4 → Al2 (SO4) 3 + 3H2O
With an excess of polybasic acid, an acid salt is formed
Al2O3 + 6H3PO4 (g) → 2Al (H2PO4) 3 + 3H2O
With an excess of oxide, a basic salt is formed
ZnO (g) + HCl → Zn (OH) Cl
Double oxides form two salts
Fe3O4 + 8HCl → FeCl2 + 2FeCl3 + 4H2O
3. Interaction with acidic oxide
amphoteric oxide + acidic oxide → salt
Al2O3 + 3SO3 → Al2 (SO4) 3
4. Interaction with alkali
amphoteric oxide + alkali → salt + water
During fusion, medium salt and water are formed, and in solution - complex salt
ZnO + 2NaOH (tv) (t) → Na2ZnO2 + H2O
ZnO + 2NaOH + H2O → Na2
5. Interaction with basic oxide
amphoteric oxide + basic oxide (t) → salt
ZnO + K2O (t) → K2ZnO2
6. Interaction with salts
amphoteric oxide + salt (t) → salt + volatile acidic oxide
Amphoteric oxides displace volatile acid oxides from their salts during fusion
Al2O3 + K2CO3 (t) → KAlO2 + CO2
Fe2O3 + Na2CO3 (t) → 2NaFeO2 + CO2
Chemical properties of bases
Bases are substances that include a metal cation and a hydroxide anion. Bases are soluble (alkalis - NaOH, KOH, Ba (OH) 2) and insoluble (Al2O3, Mg (OH) 2).
1. Soluble base + indicator → color change
When the indicator is added to the base solution, its color changes:
Colorless phenolphthalein - raspberry
Purple litmus - blue
Methyl orange - yellow
2. Interaction with acid (neutralization reaction)
base + acid → salt + water
By the reaction, medium, acidic or basic salts can be obtained. With an excess of a polyacidic acid, an acidic salt is formed, with an excess of a polyacidic base, a basic salt is formed.
Mg (OH) 2 + H2SO4 → MGSO4 + 2H2O
Mg (OH) 2 + 2H2SO4 → MG (HSO4) 2 + 2H2O
2Mg (OH) 2 + H2SO4 → (MgOH) 2SO4 + 2H2O
3. Interaction with acidic oxides
base + acid oxide → salt + water
6NH4OH + P2O5 → 2 (NH4) 3PO4 + 3H2O
4. Interaction of alkali with amphoteric hydroxide
alkali + amphoteric hydroxide → salt + water
In this reaction, amphoteric hydroxide exhibits acidic properties. During the reaction in the melt, average salt and water are obtained, and in the solution, a complex salt is obtained. Iron (III) and chromium (III) hydroxides dissolve only in concentrated alkali solutions.
2KOH (tv) + Zn (OH) 2 (t) → K2ZnO2 + 2H2O
KOH + Al (OH) 3 → K
3NaOH (conc) + Fe (OH) 3 → Na3
5. Interaction with amphoteric oxide
alkali + amphoteric oxide → salt + water
2NaOH (s) + Al2O3 (t) → 2NaAlO2 + H2O
6NaOH + Al2O3 + 3H2O → 2Na3
6. Interaction with salt
An ion exchange reaction occurs between the base and the salt. It occurs only during the precipitation of a precipitate or during the evolution of gas (with the formation of NH4OH).
A. Reaction between soluble base and soluble acid salt
soluble base + soluble acid salt → medium salt + water
If the salt and base are formed by different cations, then two middle salts are formed. In the case of acidic ammonium salts, excess alkali leads to the formation of ammonium hydroxide.
Ba (OH) 2 + Ba (HCO3) 2 → 2BaCO3 ↓ + 2H2O
2NaOH (g) + NH4HS → Na2S + NH4OH + H2O
B. Reaction of a soluble base with a soluble medium or basic salt.
Several scenarios are possible
soluble base + soluble medium / basic salt → insoluble salt ↓ + base
→ salt + insoluble base ↓
→ salt + weak electrolyte NH4OH
→ there is no reaction
Reactions occur between soluble bases and a medium salt only if the result is an insoluble salt, or an insoluble base, or a weak electrolyte NH4OH
NaOH + KCl ≠ the reaction does not go
If the original salt is formed by a multi-acid base, with a lack of alkali, a basic salt is formed
Under the action of alkalis on silver and mercury (II) salts, not their hydroxides are released, which dissolve at 25C, but insoluble oxides Ag2O and HgO.
7. Decomposition at temperature
basic hydroxide (t) → oxide + water
Ca (OH) 2 (t) → CaO + H2O
NaOH (t) ≠
Some bases (AgOH, Hg (OH) 2 and NH4OH) decompose even at room temperature
LiOH (t) → Li2O + H2O
NH4OH (25C) → NH3 + H2O
8. Interaction of alkali and transition metal
alkali + transition metal → salt + H2
2Al + 2KOH + 6H2O → 2K + 3H2
Zn + 2NaOH (s) (t) → Na2ZnO2 + H2
Zn + 2NaOH + 2H2O → Na2 + H2
9. Interaction with non-metals
Alkalis interact with some non-metals - Si, S, P, F2, Cl2, Br2, I2. In this case, two salts are often formed as a result of disproportionation.
Si + 2KOH + H2O → K2SiO3 + 2H2
3S + 6KOH (t) → 2K2S + K2SO3 + 3H2O
Cl2 + 2KOH (conc) → KCl + KClO + H2O (for Br, I)
3Cl2 + 6KOH (conc) (t) → 5KCl + KClO3 + 3H2O (for Br, I)
Cl2 + Ca (OH) 2 → CaOCl2 + H2O
4F2 + 6NaOH (decomp) → 6NaF + OF2 + O2 + 3H2O
4P + 3NaOH + 3H2O → 3NaH2PO2 + PH3
Hydroxides with reducing properties can be oxidized by oxygen
4Fe (OH) 2 + O2 + 2H2O → 4Fe (OH) 3 (= Cr)
Chemical properties of acids
1. Change the color of the indicator
soluble acid + indicator → color change
Violet litmus and methyl orange turn red, phenolphthalein becomes transparent
2. Interaction with bases (neutralization reaction)
acid + base → salt + water
H2SO4 + Mg (OH) 2 → MgSO4 + 2H2O
3. Interaction with basic oxide
acid + basic oxide → salt + water
2HCl + CuO → CuCl2 + H2O
4. Interaction with amphoteric hydroxides with the formation of medium, acidic or basic salts
acid + amphoteric hydroxide → salt + water
2HCl + Be (OH) 2 → BeCl2 + 2H2O
H3PO4 () + Zn (OH) 2 → ZNHPO4 + 2H2O
HCl + Al (OH) 3 () → Al (OH) 2Cl + H2O
5. Interaction with amphoteric oxides
acid + amphoteric oxide → salt + water
H2SO4 + ZnO → ZnSO4 + H2O
6. Interaction with salts
General reaction scheme: acid + salt → salt + acid
An ion exchange reaction takes place, which goes to the end only in the case of gas formation or precipitation.
For example: HCl + AgNO3 → AgCl ↓ + HNO3
2HBr + K2SiO3 → 2KBr + H2SiO3 ↓
A. Reaction with a salt of a more volatile or weaker acid to form a gas
HCl + NaHS → NaCl + H2S
B. Reaction between a strong acid and a strong or moderate acid salt to form an insoluble salt
strong acid + strong / medium acid salt → insoluble salt + acid
Non-volatile phosphoric acid displaces strong, but volatile hydrochloric and nitric acids from their salts, subject to the formation of an insoluble salt
B. Interaction of an acid with a basic salt of the same acid
acid1 + basic acid salt1 → medium salt + water
HCl + Mg (OH) Cl → MgCl2 + H2O
D. The interaction of a polybasic acid with a medium or acidic salt of the same acid with the formation of an acidic salt of the same acid containing a greater number of hydrogen atoms
polybasic acid1 + medium / acidic acid salt1 → acidic acid1
H3PO4 + Ca3 (PO4) 2 → 3CaHPO4
H3PO4 + CaHPO4 → Ca (H2PO4) 2
E. Interaction of hydrogen sulfide acid with salts of Ag, Cu, Pb, Cd, Hg with the formation of insoluble sulfide
acid H2S + salt Ag, Cu, Pb, Cd, Hg → Ag2S / CuS / PbS / CdS / HgS ↓ + acid
H2S + CuSO4 → CuS ↓ + H2SO4
E. Reaction of an acid with a medium or complex salt with an amphoteric metal in the anion
a) in the case of a lack of acid, a medium salt and amphoteric hydroxide are formed
acid + medium / complex salt in amphoteric metal in anion → medium salt + amphoteric hydroxide
b) in the case of an excess of acid, two average salts and water are formed
acid + medium / complex salt with amphoteric metal in the anion → medium salt + medium salt + water
G. In some cases, acids with salts enter into redox reactions or complexation reactions:
H2SO4 (conc) and I‾ / Br‾ (products H2S and I2 / SO2 and Br2)
H2SO4 (conc) and Fe² + (products SO2 and Fe³ +)
HNO3 diluted / conc and Fe² + (products NO / NO2 and Fe³ +)
HNO3 open / conc and SO3²‾ / S²‾ (products NO / NO2 and SO4²‾ / S or SO4²‾)
HClconc and KMnO4 / K2Cr2O7 / KClO3 (products Cl2 and Mn² + / Cr² + / Cl‾)
3. Interaction of concentrated sulfuric acid with solid salt
Non-volatile acids can displace volatile acids from their solid salts
7. Interaction of acid with metal
A. Interaction of acid with metals in a row before or after hydrogen
acid + metal up to Н2 → silt metal in the minimum oxidation state + Н2
Fe + H2SO4 (diluted) → FeSO4 + H2
acid + metal after H2 ≠ the reaction does not go
Cu + H2SO4 (decomp) ≠
B. Interaction of concentrated sulfuric acid with metals
H2SO4 (conc) + Au, Pt, Ir, Rh, Ta ≠ the reaction is not proceeding
H2SO4 (conc) + alkali / alkaline earth metal and Mg / Zn → H2S / S / SO2 (depending on conditions) + metal sulfate in the maximum oxidation state + H2O
Zn + 2H2SO4 (conc) (t1) → ZnSO4 + SO2 + 2H2O
3Zn + 4H2SO4 (end) (t2> t1) → 3ZnSO4 + S ↓ + 4H2O
4Zn + 5H2SO4 (end) (t3> t2) → 4ZnSO4 + H2S + 4H2O
H2SO4 (conc) + other metals → SO2 + metal sulfate in the maximum oxidation state + H2O
Cu + 2H2SO4 (conc) (t) → CuSO4 + SO2 + 2H2O
2Al + 6H2SO4 (conc) (t) → Al2 (SO4) 3 + 3SO2 + 6H2O
B. Reaction of concentrated nitric acid with metals
HNO3 (conc) + Au, Pt, Ir, Rh, Ta, Os ≠ the reaction does not proceed
HNO3 (conc) + Pt ≠
HNO3 (conc) + alkali / alkaline earth metal → N2O + metal nitrate in the maximum oxidation state + H2O
4Ba + 10HNO3 (conc) → 4Ba (NO3) 2 + N2O + 5H2O
HNO3 (conc) + other metals at temperature → NO2 + metal nitrate in the maximum oxidation state + H2O
Ag + 2HNO3 (conc) → AgNO3 + NO2 + H2O
It interacts with Fe, Co, Ni, Cr and Al only when heated, since under normal conditions these metals are passivated with nitric acid - they become chemically resistant
D. Reaction of dilute nitric acid with metals
HNO3 (decomposition) + Au, Pt, Ir, Rh, Ta ≠ the reaction does not proceed
Very passive metals (Au, Pt) can be dissolved in aqua regia - a mixture of one volume of concentrated nitric acid with three volumes of concentrated hydrochloric acid. The oxidizing agent in it is atomic chlorine, which is split off from nitrosyl chloride, which is formed as a result of the reaction: HNO3 + 3HCl → 2H2O + NOCl + Cl2
HNO3 (decomp) + alkaline / alkaline earth metal → NH3 (NH4NO3) + metal nitrate in maximum oxidation state + H2O
NH3 is converted to NH4NO3 in excess of nitric acid
4Ca + 10HNO3 (diluted) → 4Ca (NO3) 2 + NH4NO3 + 3H2O
HNO3 (broken) + metal in the series of stresses up to Н2 → NO / N2O / N2 / NH3 (depending on conditions) + metal nitrate in the maximum oxidation state + Н2О
With the rest of the metals, which stand in the range of voltages up to hydrogen and non-metals, HNO3 (diluted) forms salt, water and, mainly, NO, but, depending on the conditions, both N2O, and N2, and NH3 / NH4NO3 (the more diluted the acid , the lower the oxidation state of nitrogen in the emitted gaseous product)
3Zn + 8HNO3 (decomp) → 3Zn (NO3) 2 + 2NO + 4H2O
4Zn + 10HNO3 (decomp) → 4Zn (NO3) 2 + N2O + 5H2O
5Zn + 12HNO3 (decomp) → 5Zn (NO3) 2 + N2 + 6H2O
4Zn + 10HNO3 (fine parsed) → 4Zn (NO3) 2 + NH4NO3 + 3H2O
HNO3 (decomp) + metal after Н2 → NO + metal nitrate in the maximum oxidation state + H2O
With low-activity metals, standing after H2, HNO3 dissociates forms salt, water and NO
3Cu + 8HNO3 (decomp) → 3Cu (NO3) 2 + 2NO + 4H2O
8. Decomposition of acids at temperature
acid (t) → oxide + water
H2CO3 (t) → CO2 + H2O
H2SO3 (t) → SO2 + H2O
H2SiO3 (t) → SiO2 + H2O
2H3PO4 (t) → H4P2O7 + H2O
H4P2O7 (t) → 2HPO3 + H2O
4HNO3 (t) → 4NO2 + O2 + 2H2O
3HNO2 (t) → HNO3 + 2NO + H2O
2HNO2 (t) → NO2 + NO + H2O
3HCl (t) → 2HCl + HClO3
4H3PO3 (t) → 3H3PO4 + PH3
9. Interaction of acid with non-metals (redox reaction). In this case, the non-metal is oxidized to the corresponding acid, and the acid is reduced to a gaseous oxide: H2SO4 (conc) - to SO2; HNO3 (conc) - up to NO2; HNO3 (diluted) - to NO.
S + 2HNO3 (decomp) → H2SO4 + 2NO
S + 6HNO3 (conc) → H2SO4 + 6NO2 + 2H2O
S + 2H2SO4 (conc) → 3SO2 + CO2 + 2H2O
C + 2H2SO4 (conc) → 2SO2 + CO2 + 2H2O
C + 4HNO3 (conc) → 4NO2 + CO2 + 2H2O
P + 5HNO3 (decomp) + 2H2O → 3H3PO4 + 5NO
P + 5HNO3 (conc) → HPO3 + 5NO2 + 2H2O
H2S + G2 → 2HG + S ↓ (except F2)
H2SO3 + G2 + H2O → 2HG + H2SO4 (except F2)
2H2S (aq) + O2 → 2H2O + 2S ↓
2H2S + 3O2 → 2H2O + 2SO2 (combustion)
2H2S + O2 (short) → 2H2O + 2S ↓
More active halogens displace less active ones from NG acids (exception: F2 reacts with water, not acid)
2HBr + Cl2 → 2HCl + Br2 ↓
2HI + Cl2 → 2HCl + I2 ↓
2HI + Br2 → 2HBr + I2 ↓
10. Redox reactions between acids
H2SO4 (conc) 2HBr → Br2 ↓ + SO2 + 2H2O
H2SO4 (conc) + 8HI → 4I2 ↓ + H2S + 4H2O
H2SO4 (conc) + HCl ≠
H2SO4 (conc) + H2S → S ↓ + SO2 + 2H2O
3H2SO4 (conc) + H2S → 4SO2 + 4H2O
H2SO3 + 2H2S → 3S ↓ + 3H2O
2HNO3 (conc) + H2S → S ↓ + 2NO2 + 2H2O
2HNO3 (conc) + SO2 → H2SO4 + 2NO2
6HNO3 (conc) + HI → HIO3 + 6NO2 + 3H2O
2HNO3 (conc) + 6HCl → 3Cl2 + 2NO + 4H2O
Chemical properties of amphoteric hydroxides
1. Interaction with basic oxide
amphoteric hydroxide + basic oxide → salt + water
2Al (OH) 3 + Na2O (t) → 2NaAlO2 + 3H2O
2. Interaction with amphoteric or acidic oxide
amphoteric hydroxide + amphoteric / acidic oxide ≠ no reaction
Some amphoteric oxides (Be (OH) 2, Zn (OH) 2, Pb (OH) 2) react with acidic oxide CO2 to form precipitates of basic salts and water
2Be (OH) 2 + CO2 → (BeOH) 2CO3 ↓ + H2O
3. Interaction with alkali
amphoteric hydroxide + alkali → salt + water
Zn (OH) 2 + 2KOH (tv) (t) → K2ZnO2 + 2H2O
Zn (OH) 2 + 2KOH → K2
4. Does not interact with insoluble bases or amphoteric hydroxides
amphoteric hydroxide + insoluble base / amphoteric hydroxide ≠ no reaction
5. Interaction with acids
amphoteric hydroxide + acid → salt + water
Al (OH) 3 + 3HCl → AlCl3 + 3H2O
6. Do not react with salts
amphoteric hydroxide + salt ≠ no reaction
7. Do not react with metals / non-metals (simple substances)
amphoteric hydroxide + metal / non-metal ≠ no reaction
8. Thermal decomposition
amphoteric hydroxide (t) → amphoteric oxide + water
2Al (OH) 3 (t) → Al2O3 + 3H2O
Zn (OH) 2 (t) → ZnO + H2O
General information about salts
Let's imagine that we have an acid and an alkali, we carry out a neutralization reaction between them and get an acid and a salt.
NaOH + HCl → NaCl (sodium chloride) + H2O
It turns out that the salt consists of a metal cation and an acid residue anion.
Salts are:
1. Acidic (with one or two hydrogen cations (that is, they have an acidic (or slightly acidic) environment) - KHCO3, NaHSO3).
2. Medium (I have a metal cation and an acid residue anion, the medium must be determined using a pH meter - BaSO4, AgNO3).
3. Basic (have a hydroxide ion, that is, an alkaline (or weakly alkaline) medium - Cu (OH) Cl, Ca (OH) Br).
There are also double salts that form cations of two metals (K) upon dissociation.
Salts, with a few exceptions, are crystalline solids with high melting points. Most of the salts are white (KNO3, NaCl, BaSO4, etc.). Some salts are colored (K2Cr2O7 - orange, K2CrO4 - yellow, NiSO4 - green, CoCl3 - pink, CuS - black). According to their solubility, they can be divided into soluble, slightly soluble and practically insoluble. Acidic salts are generally better soluble in water than the corresponding average, and basic ones are worse.
Chemical properties of salts
1. Salt + water
When many salts are dissolved in water, their partial or complete decomposition occurs - hydrolysis... Some salts form crystalline hydrates. When medium salts containing an amphoteric metal in the anion are dissolved in water, complex salts are formed.
NaCl + H2O → NaOH + HCl
Na2ZnO2 + 2H2O = Na2
2. Salt + Basic oxide ≠ the reaction does not go
3. Salt + amphoteric oxide → (t) acid volatile oxide + salt
Amphoteric oxides displace volatile acid oxides from their salts during fusion.
Al2O3 + K2CO3 → KAlO2 + CO2
Fe2O3 + Na2CO3 → 2NaFeO2 + CO2
4. Salt + acidic non-volatile oxide → acidic volatile oxide + salt
Non-volatile acidic oxides displace volatile acidic oxides from their salts upon fusion.
SiO2 + CaCO3 → (t) CaSiO3 + CO2
P2O5 + Na2CO3 → (t) 2Na3PO4 + 3CO2
3SiO2 + Ca3 (PO4) 2 → (t) 3CaSiO3 + P2O5
5. Salt + base → base + salt
Reactions between salts and bases are ion exchange reactions. Therefore, under normal conditions, they proceed only in solutions (and the salt and base must be soluble) and only under the condition that a precipitate or a weak electrolyte (H2O / NH4OH) is formed as a result of exchange; gaseous products are not formed in these reactions.
A. Soluble base + soluble acidic salt → medium salt + water
If the salt and the base are formed by different cations, then two average salts are formed; in the case of acidic ammonium salts, an excess of alkali leads to the formation of ammonium hydroxide.
Ba (OH) 2 + Ba (HCO3) → 2BaCO3 + 2H2O
2KOH + 2NaHCO3 → Na2CO3 + K2CO3 + 2H2O
2NaOH + 2NH4HS → Na2S + (NH4) 2S + 2H2O
2NaOH (g) + NH4Hs → Na2S + NH4OH + H2O
B. Soluble base + soluble medium / basic salt → insoluble salt ↓ + base
Soluble base + soluble medium / basic salt → salt + insoluble base ↓
Soluble base + soluble medium / basic salt → salt + weak electrolyte NH4OH
Soluble base + soluble medium / basic salt → no reaction
The reaction between soluble bases and an average / basic salt occurs only if, as a result of ion exchange, an insoluble salt, or an insoluble base, or a weak electrolyte NH4OH is formed.
Ba (OH) 2 + Na2SO4 → BaSO4 ↓ + 2NaOH
2NH4OH + CuCl2 → 2NH4Cl + Cu (OH) 2 ↓
Ba (OH) 2 + NH4Cl → BaCl2 + NH4OH
NaOH + KCl ≠
If the starting salt is formed by a polyacid base, a base salt is formed when there is a lack of alkali.
NaOH (short) + AlCl3 → Al (OH) Cl2 + NaCl
Under the action of alkalis on silver and mercury (II) salts, not AgOH and Hg (OH) 2 are released, which decompose at room temperature, but insoluble oxides Ag2O and HgO.
2AgNO3 + 2NaOH → Ag2O ↓ 2NaNO3 + H2O
Hg (NO3) 2 + 2KOH → HgO ↓ + 2KNO3 + H2O
6. Salt + amphoteric hydroxide → the reaction does not go
7. Salt + acid → acid + salt
Primarily. the reactions of acids with salts are reactions of ion exchange, therefore they occur in solutions and only if, in this case, an acid-insoluble salt or a weaker and volatile acid is formed.
HCl + AgNO3 → AgCl ↓ + HNO3
2HBr + K2SiO3 → 2KBr + H2SiO3 ↓
2HNO3 + Na2CO3 → 2NaNO3 + H2O + CO2
A. Acid1 + salt of more volatile / weak acid2 → salt of acid1 + more volatile / weak acid2
Acids interact with solutions of salts of weaker or volatile acids. Regardless of the salt composition (medium, acidic, basic), as a rule, a medium salt and a weaker volatile acid are formed.
2CH3COOH + Na2S → 2CH3COONa + H2S
HCl + NaHS → NaCl + H2S
B. Strong acid + strong / medium acid salt → insoluble salt ↓ + acid
Strong acids interact with solutions of salts of other strong acids to form an insoluble salt. Non-volatile Н3РО4 (medium strength acid) displaces strong, but volatile hydrochloric HCl and nitric HNO3 acids from their salts, provided that an insoluble salt is formed.
H2SO4 + Ca (NO3) 2 → CaSO4 ↓ + 2HNO3
2H3PO4 + 3CaCl2 → Ca3 (PO4) 2 ↓ + 6HCl
H3PO4 + 3AgNO3 → Ag3PO4 ↓ + 3HNO3
B. Acid1 + basic acid salt1 → medium salt + water
When an acid acts on a basic salt of the same acid, a medium salt and water are formed.
HCl + Mg (OH) Cl → MgCl2 + H2O
D. Polybasic acid1 + medium / acidic acid salt1 → acidic acid1
When a polybasic acid acts on an average salt of the same acid, an acid salt is formed, and when an acid salt is acted upon, an acid salt is formed containing a greater number of hydrogen atoms.
H3PO4 + Ca3 (PO4) → 3CaHPO4
H3PO4 + CaHPO4 → Ca (H2PO4) 2
CO2 + H2O + CaCO3 → Ca (HCO3) 2
E. Acid H2S + salt Ag, Cu, Pb, Cd, Hg → Ag2S / CuS / PbS / CdS / HgS ↓ + acid
Weak and volatile hydrogen sulfide acid H2S displaces even strong acids from solutions of Ag, Cu, Pb, Cd and Hg salts, forming sulfide precipitates with them, insoluble not only in water, but also in the resulting acid.
H2S + CuSO4 → CuS ↓ + H2SO4
E. Acid + medium / complex salt with amphoteric Me in the anion → medium salt + amphoteric hydroxide ↓
→ medium salt + medium salt + H2O
When an acid acts on a medium or complex salt with an amphoteric metal in the anion, the salt is destroyed and forms:
a) in case of lack of acid - medium salt and amphoteric hydroxide
b) in the case of an excess of acid - two medium salts and water
2HCl (weeks) + Na2ZnO2 → 2NaCl + Zn (OH) 2 ↓
2HCl (week) + Na2 → 2NaCl + Zn (OH) 2 ↓ + 2H2O
4HCl (g) + Na2ZnO2 → 2NaCl + ZnCl2 + 2H2O
4HCl (g) + Na2 → 2NaCl + ZnCl2 + 4H2O
It should be borne in mind that in some cases, ORP or complexation reactions occur between acids and salts. So, the OVR is joined by:
H2SO4 conc. and I‾ / Br‾ (products H2S and I2 / SO2 and Br2)
H2SO4 conc. and Fe² + (products SO2 and Fe³ + )
HNO3 dil. / Conc. and Fe² + (products NO / NO2 and Fe 3 + )
HNO3 dil. / Conc. and SO3²‾ / S²‾ (NO / NO2 products and sulfate / sulfur or sulfate)
HCl conc. and KMnO4 / K2Cr2O7 / KClO3 (products are chlorine (gas) and Mn²+ / Cr³ + / Cl‾.
G. The reaction takes place without solvent.
Sulfuric acid conc. + salt (TV) → acidic / medium salt + acidic
Non-volatile acids can displace volatile acids from their dry salts. Most often, the interaction of concentrated sulfuric acid with dry salts of strong and weak acids is used, with the formation of an acid and an acidic or medium salt.
H2SO4 (conc) + NaCl (tv) → NaHSO4 + HCl
H2SO4 (conc) + 2NaCl (tv) → Na2SO4 + 2HCl
H2SO4 (conc) + KNO3 (tv) → KHSO4 + HNO3
H2SO4 (conc) + CaCO3 (tv) → CaSO4 + CO2 + H2O
8. Soluble salt + soluble salt → insoluble salt ↓ + salt
Reactions between salts are exchange reactions. Therefore, under normal conditions, they proceed only if:
a) both salts are soluble in water and taken in the form of solutions
b) as a result of the reaction, a precipitate or a weak electrolyte is formed (the latter is very rare).
AgNO3 + NaCl → AgCl ↓ + NaNO3
If one of the starting salts is insoluble, the reaction proceeds only when an even more insoluble salt is formed as a result. The criterion for "insolubility" is the value of PR (solubility product), however, since its study is beyond the scope of the school course, the cases when one of the reagent salts is insoluble are not further considered.
If a salt is formed in the exchange reaction, which completely decomposes as a result of hydrolysis (in the solubility table there are dashes in place of such salts), then the products of the hydrolysis of this salt become the reaction products.
Al2 (SO4) 3 + K2S ≠ Al2S3 ↓ + K2SO4
Al2 (SO4) 3 + K2S + 6H2O → 2Al (OH) 3 ↓ + 3H2S + K2SO4
FeCl3 + 6KCN → K3 + 3KCl
AgI + 2KCN → K + KI
AgBr + 2Na2S2O3 → Na3 + NaBr
Fe2 (SO4) 3 + 2KI → 2FeSO4 + I2 + K2SO4
NaCl + NaHSO4 → (t) Na2SO4 + HCl
Medium salts sometimes interact with each other to form complex salts. OVR is possible between salts. Some salts interact when fusing.
9. Salt of less active metal + metal more active → metal less active ↓ + salt
The more active metal displaces the less active metal (standing to the right in the series of stress) from the solution of its salt, while a new salt is formed, and the less active metal is released in a free form (settles on the plate of the active metal). The exception is that alkali and alkaline earth metals in solution interact with water.
Salts with oxidizing properties in solution enter into other redox reactions with metals.
FeSO4 + Zn → Fe ↓ + ZnSO4
ZnSO4 + Fe ≠
Hg (NO3) 2 + Cu → Hg ↓ + Cu (NO3) 2
2FeCl3 + Fe → 3FeCl2
FeCl3 + Cu → FeCl2 + CuCl2
HgCl2 + Hg → Hg2Cl2
2CrCl3 + Zn → 2CrCl2 + ZnCl2
Metals can displace each other from molten salts (the reaction is carried out without air access). It should be remembered that:
a) when melted, many salts decompose
b) the series of stress of metals determines the relative activity of metals only in aqueous solutions (for example, Al in aqueous solutions is less active than alkaline earth metals, and in melts it is more active)
K + AlCl3 (melt) → (t) 3KCl + Al
Mg + BeF2 (melt) → (t) MgF2 + Be
2Al + 3CaCl2 (melt) → (t) 2AlCl3 + 3Ca
10. Salt + non-metal
Reactions of salts with non-metals are few. These are redox reactions.
5KClO3 + 6P → (t) 5KCl + 3P2O5
2KClO3 + 3S → (t) 2KCl + 2SO2
2KClO3 + 3C → (t) 2KCl + 3CO2
More active halogens displace less active halogen salts from solutions. An exception is molecular fluorine, which in solutions reacts not with salt, but with water.
2FeCl2 + Cl2 → (t) 2FeCl3
2NaNO2 + O2 → 2NaNO3
Na2SO3 + S → (t) Na2S2O3
BaSO4 + 2C → (t) BaS + 2CO2
2KClO3 + Br2 → (t) 2KBrO3 + Cl2 (the same reaction is typical for iodine)
2KI + Br2 → 2KBr + I2 ↓
2KBr + Cl2 → 2KCl + Br2 ↓
2NaI + Cl2 → 2NaCl + I2 ↓
11. Decomposition of salts.
Salt → (t) thermal decomposition products
1. Salts of nitric acid
The products of thermal decomposition of nitrates depend on the position of the metal cation in the series of metal stresses.
MeNO3 → (t) (for Me to the left of Mg (excluding Li)) MeNO2 + O2
MeNO3 → (t) (for Me from Mg to Cu, as well as Li) MeO + NO2 + O2
MeNO3 → (t) (for Me to the right of Cu) Me + NO2 + O2
(during the thermal decomposition of iron (II) / chromium (II) nitrate, iron (III) / chromium (III) oxide is formed.
2. Ammonium salts
All ammonium salts decompose on ignition. Most often, this produces ammonia NH3 and acid or its decomposition products.
NH4Cl → (t) NH3 + HCl (= NH4Br, NH4I, (NH4) 2S)
(NH4) 3PO4 → (t) 3NH3 + H3PO4
(NH4) 2HPO4 → (t) 2NH3 + H3PO4
NH4H2PO4 → (t) NH3 + H3PO4
(NH4) 2CO3 → (t) 2NH3 + CO2 + H2O
NH4HCO3 → (t) NH3 + CO2 + H2O
Sometimes ammonium salts containing oxidizing anions decompose on heating with the release of N2, NO or N2O.
(NH4) Cr2O7 → (t) N2 + Cr2O3 + 4H2O
NH4NO3 → (t) N2O + 2H2O
2NH4NO3 → (t) N2 + 2NO + 4H2O
NH4NO2 → (t) N2 + 2H2O
2NH4MnO4 → (t) N2 + 2MnO2 + 4H2O
3. Salts of carbonic acid
Almost all carbonates decompose to metal oxide and CO2. Alkali metal carbonates other than lithium do not decompose when heated. Silver and mercury carbonates decompose to free metal.
MeCO3 → (t) MeO + CO2
2Ag2CO3 → (t) 4Ag + 2CO2 + O2
All hydrocarbons are decomposed to the corresponding carbonate.
MeHCO3 → (t) MeCO3 + CO2 + H2O
4. Sulfurous acid salts
When heated, sulfites disproportionate, forming sulfide and sulfate. The sulfide (NH4) 2S formed during the decomposition of (NH4) 2SO3 immediately decomposes into NH3 and H2S.
MeSO3 → (t) MeS + MeSO4
(NH4) 2SO3 → (t) 2NH3 + H2S + 3 (NH4) 2SO4
Hydrosulfites decompose to sulfites, SO2 and H2O.
MeHSO3 → (t) MeSO3 + SO2 + H2O
5. Sulfuric acid salts
Many sulfates decompose at t> 700-800 C to metal oxide and SO3, which decomposes to SO2 and O2 at this temperature. Sulfates of alkali metals are heat-resistant. Silver and mercury sulfates decompose to free metal. Hydrosulfates decompose first to disulfates and then to sulfates.
2CaSO4 → (t) 2CaO + 2SO2 + O2
2Fe2 (SO4) 3 → (t) 2Fe2O3 + 6SO2 + 3O2
2FeSO4 → (t) Fe2O3 + SO3 + SO2
Ag2SO4 → (t) 2Ag + SO2 + O2
MeHSO4 → (t) MeS2O7 + H2O
MeS2O7 → (t) MeSO4 + SO3
6. Complex salts
Hydroxo complexes of amphoteric metals decompose mainly into medium salt and water.
K → (t) KAlO2 + 2H2O
Na2 → (t) ZnO + 2NaOH + H2O
7. Basic salts
Many basic salts decompose when heated. Basic salts of anoxic acids decompose into water and oxosalts
Al (OH) 2Br → (t) AlOBr + H2O
2AlOHCl2 → (t) Al2OCl4 + H2O
2MgOHCl → (t) Mg2OCl2 + H2O
Basic salts of oxygen-containing acids decompose into metal oxide and thermal decomposition products of the corresponding acid.
2AlOH (NO3) 2 → (t) Al2O3 + NO2 + 3O2 + H2O
(CuOH) 2CO3 → (t) 2CuO + H2O + CO2
8. Examples of thermal decomposition of other salts
4K2Cr2O7 → (t) 4K2CrO4 + 2Cr2O3 + 3O2
2KMnO4 → (t) K2MnO4 + MnO2 + O2
KClO4 → (t) KCl + O2
4KClO3 → (t) KCl + 3KClO4
2KClO3 → (t) 2KCl + 3O2
2NaHS → (t) Na2S + H2S
2CaHPO4 → (t) Ca2P2O7 + H2O
Ca (H2PO4) 2 → (t) Ca (PO3) 2 + 2H2O
2AgBr → (hν) 2Ag + Br2 (= AgI)
Most of the material presented was taken from the manual by N.E.Deryabina. "Chemistry. The main classes of inorganic substances". IPO "At Nikitskiye Vorota" Moscow 2011.