Diffusion in home experiments. Diffusion
Water vapor occurs when water boils and evaporates at different temperatures. For the transition of water into a gaseous state, heat is absorbed from the environment in an amount of about 600 kcal / kg. Water vapor in the air is not noticeable ("clouds of water vapor" are water droplets floating in the air).
Only a certain amount of water vapor can be in the air: the warmer the air, the greater the possible content of water vapor. The percentage of vapor in the air actually determines the relative humidity of the air. With a decrease in air temperature and the content of water vapor remains unchanged, the relative humidity of the air increases.
Example: the content of water vapor in the air is 125.2 kg/m2.
|
If, in this example, the air temperature is further lowered, then the water vapor condenses into a liquid. The temperature at which the relative humidity of the air reaches 100% is called the dew point of the mixture of air and water vapor.
Atmospheric air pressure 1 atm is equal to 10,000 kg/m2; In a mixture of air and water vapor, part of the pressure is caused by water vapor. It is advisable to use such an indicator to characterize the content of water vapor in the air, since the possibilities of diffusion are more obvious (0.06 g of water / 1 kg of air = 1 kg / m2). Therefore, the difference in water vapor pressure (Fig. 3) reflects only the different content of water vapor molecules at the same total pressure of the air mixture; in contrast, the absolute pressure difference is as in a steam boiler (Fig. 4), for example, in the bubbles of roofing carpets.
Different water vapor pressures can be equalized by diffusion through the structural elements and their layers. Diffusion resistance is characterized by the coefficient μd(cm, m). If an air gap is taken into account, then the diffusion resistance coefficient is determined from the table "Thermal resistance and diffusion resistance coefficients of building materials".
When diffusing inside building structures, areas with reduced pressure appear. Similarly to the distribution of temperature in the structure, the pressure is distributed in the individual layers in accordance with their share in the total coefficient of resistance to diffusion. Air gaps of small thickness (outside 0.5, inside 2 cm) can be ignored.
Example.
| Inside 20°/50% = H 9 kg/m2; outside 15°/80% = 14kg/m2. Wall thickness 24cm: μd= 4.5 x 24 = 108 cm. Plaster from the inside 1.5 cm: μd= 6 x 15 = 6 cm | Difference 119 - 14 \u003d 105 kg / m294.7% x 105 \u003d 9.95 kg / m2 5.3% x 105 = 5.5 kg/m2 |
| 114 cm | 100% |
Diffusion examples.
To prevent the destruction of building structures, it is necessary to exclude moisture condensation in them. Condensation occurs where the actual water vapor content threatens to exceed the amount corresponding to the temperature. In the examples in Fig. 5-10 the structure with boundary air layers is shown on a scale proportional to their thermal insulation. The curve next to the temperature ramp shows the maximum possible water vapor pressure.
To prevent damage, it is important to consider: sufficient thermal insulation. The example (fig. 5) shows a single layer construction without condensation. In the example (Fig. 6), condensation occurs on the inside of the structure, since the proportion of the boundary air layer is too large. The boundary air layer should not exceed a certain value x in the heat transfer resistance 1/k (Table 2);
correct layering. The diffusion curve should have as steep a slope as possible inside and be flat outside (Fig. 7). Otherwise, condensation occurs (Fig. 8). Slope is characterized by μd coefficient: inside high diffusion resistance coefficient, good thermal conductivity = high μd coefficient; outside low diffusion resistance coefficient, poor thermal conductivity = low μd coefficient;
the correct location of the vapor barrier. If the vapor barrier is outside, then the water vapor pressure drops there and condensation forms as a result (Fig. 9).
To avoid this, the vapor barrier layer must be located inside, and the layers in front of it must not exceed the x value in the total heat transfer resistance 1/k (Table 2).
Table 1. Water vapor pressure in air.
|
Table 2. Maximum proportion of the boundary air layer before the vapor barrier (x).
|
|||||||||||||||||||||||||||||||||||||
| 1. The content of water vapor in the air at different relative humidity. 2. In accordance with the distribution of temperature in the building structure, there is a curve for the maximum content of water vapor in the air diffusing through the structure - the saturation pressure curve. |
|
 |
3. Relative steam pressure difference on both sides of the building structure. 4. Absolute steam pressure difference on both sides of the building structure. |
 |
5. Water vapor pressure remains below the maximum possible - no condensate. 6. The boundary air layer is too large due to insufficient thermal insulation: condensate on the structure and inside it: X is the maximum allowable thickness of the boundary air layer. |
 |
7. Coefficient characterizing the arrangement of layers: the steepness of the curve decreases towards the outside - good. 8. Incorrect arrangement of layers: the coefficient and the steepness of the curve increase towards the outside, as a result of which condensation forms inside the structure. |
 |
9. Vapor barrier on the cold side: condensate inside the structure. 10. Additional vapor barrier on the warm side prevents condensation, X = maximum thermal insulation on the inside of the vapor barrier. |
Ernst Neufert. Structural design / Ernst Neufert "BAUENTWURFSLEHRE"
Let's start with the fact that the liquid is an intermediate state of aggregation. At the critical boiling point, it is similar to gases, and at low temperatures, characteristics similar to a solid appear. The liquid does not have an ideal model, which significantly complicates the description of its equilibrium thermodynamic properties, freezing point, viscosity, diffusion, thermal conductivity, surface tension, entropy, enthalpy.
Definition
What is diffusion? This is the spreading, distribution, movement of particles of the medium, which leads to the transfer of matter, the establishment of equilibrium concentrations. In the absence of external influences, this process is determined by the thermal motion of the particles. In this case, the diffusion process is directly proportional to the concentration. The diffusion flux will change similarly

Varieties
If diffusion in a liquid proceeds with a change in temperature, it is called thermal diffusion, in an electric field - electrodiffusion.
The process of movement of large particles in a liquid or gas occurs under the laws of Brownian motion.

Flow features
Diffusion in gases, liquids and solids proceeds at different rates. Due to differences in the nature of the thermal motion of particles in different media, the process has the maximum speed in gases, and the minimum rate - in solids.
The trajectory of the particle is a broken line, since the direction and speed periodically change. Due to the disorder of motion, a gradual removal of the particle from its original position is observed. Its displacement along a straight line is much shorter than the path that takes place along a broken path.

Fick's Law
Diffusion in a liquid obeys two Fick's laws:
- the diffusion flux density is directly proportional to the concentration with the diffusion coefficient;
- the rate of change in the diffusion flux density is directly proportional to the rate of change in concentration and has the opposite direction.
Diffusion in a liquid is characterized by jumps of molecules from one equilibrium position to another. Each such jump is observed when energy is imparted to the molecule in a volume sufficient to break the bond with other particles. jump does not exceed the distance between molecules.
Discussing what diffusion in a liquid is, we note that the process depends on temperature. With its increase, the structure of the liquid is “loosened”, as a result of which a sharp increase in the number of jumps per unit of time is observed.
Diffusion in gases, liquids and solids has some distinctive characteristics. For example, in solids, the mechanism is associated with the movement of atoms within the crystal lattice.

Features of the phenomenon
Diffusion in a liquid is of practical interest due to the fact that it is accompanied by the equalization of the concentration of a substance in an initially inhomogeneous medium. Much more particles escape from areas with a high concentration.
Experiments
Experiments with liquids have shown that diffusion is of particular importance in chemical kinetics. During the flow on the surface of the reactants or catalyst, this process contributes to the determination of the rate of removal of the reaction products and the addition of the initial reagents.
What explains diffusion in liquids? Solvent molecules are able to penetrate through translucent membranes, resulting in an osmotic pressure. This phenomenon has found application in chemical and physical methods for the separation of substances.

Biological systems
In this case, diffusion models can be considered on the example of air oxygen entering the lungs, absorption of digestive products from the intestine into the blood, absorption of mineral elements by root hairs. Diffusion of ions occurs during the generation of bioelectric impulses by muscle and nerve cells.
The physical factor that affects the selectivity of the accumulation of certain elements in the cells of the body is the different rate of penetration of ions through the cell membranes. This process can be expressed by Fick's law, replacing the value of the diffusion coefficient by the membrane permeability, and instead of the concentration gradient, use the difference in values on both sides of the membrane. With the diffusion penetration of water and gases into the cell, the osmotic pressure indicators outside and inside the cell change.
Analyzing what diffusion depends on, we note that there are several types of this process. The simple form is associated with the free transfer of ions and molecules towards the gradient of their electrochemical potential. For example, this option is suitable for those substances in which the molecules are of small size, for example, methyl alcohol, water.
The limited variant assumes a weak transfer of matter. For example, even small particles are not able to penetrate into the cell.
History pages
Diffusion was discovered during the heyday of ancient Greek culture. Democritus and Anaxogoras were convinced that any substance consists of atoms. They explained the variety of substances common in nature by the connections between individual atoms. They assumed that these particles could mix to form new substances. Among the founders of the molecular-kinetic theory, which explained the mechanism of diffusion, Mikhail Lomonosov played a special role. They gave a definition to a molecule, an atom, and explained the mechanism of dissolution.

Experiments
Experience with sugar allows you to understand all the features of diffusion. If you put a piece of sugar in cold tea, a thick syrup will gradually form at the bottom of the cup. It is visible to the naked eye. After some time, the syrup will be evenly distributed throughout the volume of the liquid and will no longer be visible. This process proceeds spontaneously and does not imply mixing of the components of the solution. Similarly, the fragrance of perfume spreads throughout the volume of the room.
The above experiments indicate that diffusion is a spontaneous process of penetration of molecules of one substance into another. The spread of matter occurs in all directions, despite the presence of gravity. Such a process is a direct confirmation of the constant movement of the molecules of a substance.
So, in the above example, the diffusion of sugar and water molecules is carried out, which is accompanied by a uniform distribution of organic matter molecules throughout the entire volume of the liquid.
Experiments make it possible to detect diffusion not only in liquids, but also in gaseous substances. For example, you can install a container with ether vapor on the scales. Gradually, the cups will come into balance, then the glass of ether will be heavier. What is the reason for such a phenomenon?
Over time, ether molecules mix with air particles, and a specific smell begins to be felt in the room. In a high school physics course, an experiment is considered in which a teacher dissolves a grain in water. At first, a clear trajectory of grain movement is visible, but gradually the entire solution acquires a uniform shade. Based on the experiment, the teacher explains the features of diffusion.
To identify factors that affect the rate of the process in liquids, you can use water of different temperatures. In a hot liquid, the process of mutual mixing of molecules is observed much faster, therefore, there is a direct relationship between the temperature value and the rate of diffusion.
Conclusion
Experiments carried out with gases, liquids and allow us to formulate the laws of physics, to establish the relationship between individual quantities.
It was as a result of the experiments that the mechanism of mutual penetration of particles of one substance into another was established, and the chaotic nature of their movement was proved. Empirically, it was found that diffusion occurs fastest in gaseous substances. This process is of great importance for wildlife, is used in science and technology.
Thanks to this phenomenon, the homogeneous composition of the earth's atmosphere is maintained. Otherwise, the stratification of the troposphere into separate gaseous substances would be observed, and heavy carbon dioxide, unsuitable for breathing, would be closest to the surface of our planet. What would it lead to? Wildlife would simply cease to exist.
The role of diffusion in the plant world is also great. The lush crown of trees can be explained by diffusion exchange through the surface of the leaves. As a result, not only breathing is carried out, but also the nutrition of the tree. Currently, foliar feeding of shrubs and trees is used in agriculture, which involves spraying the crown with special chemical compounds.
It is during diffusion that the plant receives nutrients from the soil. Physiological processes occurring in living organisms are also associated with this phenomenon. For example, salt balance is impossible without diffusion. Such processes are of great importance in supplying lakes and rivers with oxygen. The gas enters the depths of the reservoir precisely by diffusion. If such a process were absent, life inside the reservoir would cease to exist.
Taking medications that allow a person to protect himself from pathogens of various diseases and improve well-being is also based on diffusion. This phenomenon is used in the welding of metals, the production of sugar juice from beet chips, and the preparation of confectionery. It is difficult to find such a branch of modern industry where diffusion is not used.
Osmosis is the diffusion of water through a semi-permeable membrane separating two solutions from a lower concentration to a higher one.[ ...]
At the beginning of the third period, the diffusion of water usually occurs without much difficulty. However, as the wood dries, the diffusion rate decreases so much that a dry layer forms on the surface of the wood. Thus, the main condition on which the drying rate in the third period depends is the diffusion of water inside the dried wood. Compared to the diffusion value, the retarding role of the gas film is now negligible. Similarly, the speed of the coolant and the partial pressure of water vapor have only a secondary influence on the process.[ ...]
The nature of the disease. The disease consists in the diffusion of water from the body into the intestinal tract. The amount of this diffusible water is colossal (about 30 l/day), and therefore it is continuously excreted in the form of vomiting and loose stools. As a result, dehydration of the body occurs, the intensity of oxidative processes rapidly decreases, and the tissues are saturated with products of incomplete combustion and carbon dioxide. The incubation period is about three days.[ ...]
Osmotic pressure is the pressure that is the result of the diffusion of water through the membrane (from a lower concentration of a solution to a higher one).[ ...]
An increase in the relative number of mobile monomeric water molecules and the activity of hydroxide ions relative to hydrogen ions, apparently, causes the acceleration of water diffusion, which affects the processes of osmosis, which are of great importance for the life of plant and animal organisms.[ ...]
In other work, researchers concluded that the anion of the sulfo group in the cation exchanger binds three water molecules. Apparently, the difference in the results depends to a large extent on the difference in the methods for estimating the amount of hydration of ionized groups in the ion-exchange resin. In any case, it has been fairly accurately established that sulfocation exchangers in the H + form swell more strongly than in salt forms, while weakly acidic cation exchangers, which are practically not ionized in the H form, swell mainly in salt forms. For the same reason, weakly basic anion exchangers swell in salt forms also much more strongly than in the OH form. Non-ion exchange transfer of electrolytes towards water diffusion when osmotic equilibrium of ionite grains with an external solution in dilute solutions is established does not have any significant effect on the behavior of ion exchange resins during water desalination or regeneration of ion exchange filters. With an increase in the concentration of acids and alkalis in regeneration solutions, this non-ion-exchange transfer of electrolytes turns out to be so significant that it cannot be neglected.[ ...]
It is well known that in some hydrates there is only a ring or only a vacancy diffusion mechanism, which is not associated with disordering. In these cases, diffusion is observed, as a rule, only at high temperatures. In this crystal, water molecules are arranged in six-wheel zigzag rings, as if carved from the structure of ice. The axes of all the rings are parallel to each other, and the H-II directions form an angle of 47° with the axes of the rings. From here, according to the rules of averaging the dipole interaction, one can find the average constant of this interaction - 9 kHz. Measurements have shown that diffusion in dNoptase is observed only at temperatures above +120°C, and the characteristic frequency is precisely 9 kHz. For apophyllite, another hydrated silicate, diffusion begins at 170°C, calculation and experiment give almost identical values with a frequency characteristic of -6.5 kHz. In patrolite, water diffusion at +150°С averages the dipole-dipole interaction to zero, in complete disagreement with the expected value, due to the fact that in this crystal the angle between the H-H vectors and the symmetry axis is almost equal to the magic one.[ ...]
Champetier and Bonnet argued that cotton selectively absorbs acid. Kazbekar and Nil discovered the selective absorption of water by cellophane when swollen in acid solutions due to the faster diffusion of water in comparison with acid into the film. A detailed study of the selective absorption of water and acid has not been carried out.[ ...]
Membrane (from Latin membrana - membrane) - a thin film or plate, usually fixed along the contour; osmosis (from the Greek osmos - push, pressure) - one-way diffusion of water through a semi-permeable partition (membrane) that separates the solution from pure water or a solution of lower concentration; ultrafiltration (from lat. ultra - over, beyond) - separation of solutions and colloidal systems using semi-permeable membranes in special apparatuses under a pressure of 0.1 - 0.8 MPa.[ ...]
At temperatures above 200-250 K, the NMR spectra of wide-pore zeolites sharply (hundreds of times) narrow and acquire a structure characteristic of water diffusing in crystals. In this case, two facts are significant. First, the width of the narrowed spectrum remains constant up to the dehydration temperature (200-300°C and more). This means that at all temperatures the molecule moves along the same diffusion path, which is strictly specified by the crystal structure, in exactly the same way as in crystalline hydrates. Secondly, despite the low-temperature mobility, very high values of the dehydration temperature remain. This feature sharply distinguishes zeolites from crystalline hydrates, in which dehydration or melting rarely occurs at temperatures significantly above 100°C. The nature of the high-temperature hydrated state of zeolites became clear only after the discovery of the "two-phase" structure of zeolite water. It turned out that the diffusion of water molecules in the zeolite channels does not prevent some of these molecules from being rigidly bound in the zeolite channels. For example, in mordenite, despite the beginning of the diffusion narrowing of the NMR spectrum at –100°C, even at +100°C, about 10% of rigidly bound water remains (in this case, complete dehydration occurs only at 450°C). It was suggested that these tightly bound molecules, like plugs, block the zeolite channel, blocking the path of diffusing molecules. Hence, it is natural to put forward an isochoric model of zeolite water in a closed space of channels. Heating increases the pressure inside the channel, and along with the pressure, the “melting” temperature of the zeolite water also increases. In accordance with what has been said, the diffusion of water in hydrated zeolites can be considered as isochoric (in a closed volume) melting. It is also obvious that the effectiveness of "plugs" in blocking the volume of channels is associated with their collective properties arising from the presence of stronger water-water bonds in certain areas of zeolite channels.[ ...]
Comparison with experience both confirms and does not confirm these expectations. But for some reason, hydrates of chlorides and bromides of calcium, strontium and barium fall out of the pattern, in which, contrary to everything, water diffusion is not detected until melting.[ ...]
The possibility of using calcium and zinc ferrites in primers along with anticorrosive pigments to replace toxic and expensive pigments based on lead and chromium has been studied. Primers containing calcium and zinc ferrites present a more severe barrier to water and oxygen diffusion than iron oxide pigmented coatings. In alkyd paints, calcium ferrite is more effective. The ratio between inert pigment and calcium ferrite in primers is 60:40. In chlorinated rubber paints, zinc ferrite is more effective, and the ratio between inert pigment and zinc ferrite is 80:20-70:30. It is noted that the protective effect of calcium and zinc ferrites is weaker than that of classical anticorrosive pigments.[ ...]
The mechanism of poisoning of living organisms is better explained by another theory, according to which poisoning occurs as a result of mercury and copper ions entering the respiratory or digestive organs, as a result of which the protein of these organs coagulates and the body dies. According to this theory, the protective effect of mercury oxide and cuprous oxide is explained as follows. Due to the diffusion of sea water into the paint film, mercury oxide and cuprous oxide are exposed to NaCl contained in sea water. As a result of this action, as mentioned above, a salt of complex composition 6MaCl 3H Cb CuCl2 is formed. A solution of this salt, containing mercury and copper ions, slowly diffusing in the direction opposite to the diffusion of water, creates in the immediate vicinity of the vessel a zone that is poisonous for representatives of marine fauna. This zone becomes poisonous, as mentioned above, even with a slight content of mercury ions in the water. and copper. With this mechanism of action of mercury oxide and cuprous oxide, all the simplest animal organisms that fall into the zone poisoned by mercury and copper ions die, and only individual specimens of them can accidentally approach the ship. Continuous fouling can begin only after a significant depletion of the outer layer of paint with mercury and copper. In practice, such a course of the process of fouling of the vessel is observed - fouling begins with the settlement of individual specimens of mollusks, and continuous fouling, much less intense than when using conventional paint, begins much later than in the case of painting the ship with ordinary oil paint.
Physics is one of the most interesting, mysterious and at the same time logical sciences. She explains everything that can be explained, even how tea becomes sweet and soup becomes salty. A true physicist would say otherwise: this is how diffusion proceeds in liquids.
Diffusion
Diffusion is a magical process of penetration of the smallest particles of one substance into the intermolecular spaces of another. By the way, this penetration is mutual.
Do you know how this word is translated from Latin? Spreading, spreading.
How does diffusion occur in liquids?
Diffusion can be observed in the interaction of any substances: liquid, gaseous and solid.
To find out how diffusion proceeds in liquids, you can try throwing a few grains of paint, ground lead or, for example, potassium permanganate into a transparent vessel with clean water. It is better if this vessel is high. What will we see? First, the crystals will sink to the bottom under the action of gravity, but after a while a halo of colored water will appear around them, which will spread and spread. If we do not approach these vessels for at least a few weeks, we will find that the water is almost completely colored.
Another good example. In order for sugar or salt to dissolve faster, they must be stirred in water. But if this is not done, sugar or salt will dissolve on their own after a while: tea or compote will become sweet, and soup or brine will become salty.
How diffusion proceeds in liquids: experience
In order to determine how the diffusion rate depends on the temperature of a substance, a small but very revealing experiment can be carried out.
Take two glasses of the same volume: one with cold water, the other with hot. Pour an equal amount of instant powder (for example, coffee or cocoa) into both glasses. In one of the vessels, the powder will begin to dissolve more intensively. Do you know which one exactly? Guess? Where the water temperature is higher! After all, diffusion proceeds in the course of a random, chaotic movement of molecules, and at high temperatures this movement occurs much faster.

Diffusion can occur in any substance, only the time of the occurrence of this phenomenon differs. The highest speed is in gases. That is why you can not store butter in the refrigerator next to herring or lard, grated with finely chopped garlic. Liquids follow (from the lowest density to the highest). And the slowest is the diffusion of solids. Although at first glance there is no diffusion in solids.
Purpose of work: to prove that diffusion depends on temperature; oo consider examples of diffusion in home experiments; oo make sure that diffusion in different substances occurs in different ways.
Relevance: Diffusion proves that bodies are composed of molecules that are in random motion; diffusion is of great importance in the life of humans, animals and plants, as well as in technology
What is diffusion?
Diffusion is a spontaneous mixing of contacting substances, which occurs due to the chaotic (random) movement of molecules.
Another definition: diffusion diffusio - spreading, spreading, scattering) - the process of transferring matter or energy from an area with high concentration to an area with low concentration.
The most famous example of diffusion is the mixing of gases or liquids (if you drop ink into water, the liquid will become uniformly colored after a while).
Diffusion occurs in liquids, solids and gases. Diffusion occurs most rapidly in gases, slower in liquids, and even slower in solids, which is due to the nature of the thermal motion of particles in these media. The trajectory of movement of each particle of gas is a broken line, since during collisions the particles change the direction and speed of their movement. For centuries, workers welded metals and made steel by heating solid iron in a carbon atmosphere, without having the slightest idea of the diffusion processes taking place. Only in 1896. study of the problem began.
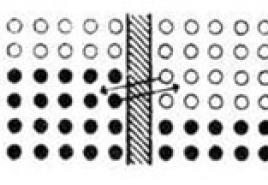
The English metallurgist William Roberts-Austin measured the diffusion of gold in lead in a simple experiment. He melted a thin disk of gold onto the end of a cylinder of pure lead 1 inch (2.45 cm) long, placed this cylinder in a furnace where the temperature was maintained at about 200C, and kept it in the furnace for 10 days. It turned out that a completely measurable amount of gold passed through the entire cylinder. This proves it again. that the rate of diffusion increases very rapidly with increasing temperature. For example, zinc diffuses into copper at 300C almost 100 million times faster than at room temperature.
Diffusion of molecules proceeds very slowly. For example, if a piece of sugar is lowered to the bottom of a glass of water and the water is not stirred, it will take several weeks before the solution becomes homogeneous.
Does diffusion depend on temperature?
The phenomenon of diffusion can be observed at home when brewing tea. During the experiment, two glasses with cold and hot water were used. When brewing tea, it was found that in a glass of hot water, the brewing process was faster.
At home, the phenomenon of diffusion manifests itself everywhere. When mom is in the kitchen cutting onions, cooking chicken, cooking dinner, or preparing a marinade for pouring vegetables, the aromas from the kitchen spread throughout the apartment.
I investigated the dependence of the rate of spread of the fragrance of perfume in a room on temperature: from one part of the room to another, the fragrance of perfume spread in 20.53 seconds. ; then I sprayed perfume near the table lamp, time - 14.03 sec.
Conclusion: The rate of diffusion increases with temperature, as the speed of movement of molecules increases.
diffusion around us.
When the rays of the sun enter the room, you can observe a peculiar >.
On this occasion, Lucretius Carus wrote:
Look here: whenever the sunlight penetrates
In our dwellings and darkness cuts through with its rays,
Many bodies in the void, you will see, flickering,
Rushing back and forth in a radiant glow of light.
As if in eternal struggle they fight in battles and battles,
All of a sudden they rush into battles in groups, not knowing peace.
Due to diffusion, indoor dust particles contain mold particles, heavy metal molecules that are found in furniture, finishing materials and other apartment items>. Easily cope with toxic substances dissolved in the air of rooms, indoor flowers: nephrolepis, dieffenbachia, spurge, ivy, pelargonium, sansevieria, etc. And all this happens due to diffusion
The well-known agave (aloe) is able to reduce the number of harmful microbes by 4 times, and the prickly pear cactus reduces the number of mold fungi in the air by 6-7 times. Tobacco smoke, linoleum coatings are harmful to our health. Indoor plants (Ficus Benjamin, Tradescantia, Chlorophytum) can absorb and decompose toxic substances.
Diffusion study in vegetables.
Experience with apples
Apples of different varieties were used: >, >, >.
In apple varieties > manganese penetration was slower. This variety of apples is winter, perhaps it is less juicy, and their structure is more dense.
Experience with vegetables
The following vegetables were used for the experiment: turnips, carrots, zucchini, potatoes
After three hours, it was found that the penetration of manganese in zucchini, potatoes was greater than in turnips and carrots. Turnips and carrots have a denser structure, and the penetration depth of manganese particles was less.
Diffusion and safety
The combustible propane gas we use at home for cooking has no color. Therefore, it would be difficult to immediately notice a gas leak. And in case of leakage due to diffusion, the gas spreads throughout the room. and we smell this leak. Meanwhile, at a certain ratio of gas to air in a closed room, a mixture is formed that can explode. For example, from a lit match. The gas can also cause poisoning of people.
Conclusions: oo During diffusion, particles of one substance penetrate into the gaps between the particles of another substance, and the substances are mixed.
oo The rate of diffusion increases with increasing temperature.
oo Diffusion is of great importance in the life processes of humans, animals and plants.