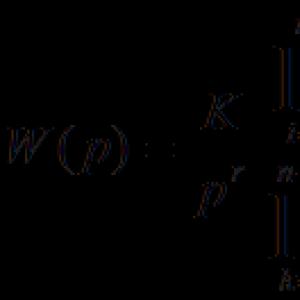Carbon equivalent. Carbon equivalent and weldability of steels Calculate the equivalent carbon content of steel 70 30xgsa
1. An indicator of the weldability of steel, expressed as a reduction to the carbon content of the sum of the concentrations of Mo, Cr, Mn, Si and other elements in the steel, which increase the stability of austenite and, accordingly, lower the temperature at which the martensitic transformation of steel begins. The International Welding Invariant equation is most often used to determine carbon equivalent (Ce):
C e \u003d C + Mn / 6 + (Cr + Mo + V) / 5 + (Cu + Ni) / 15,
where C, Mn, Cr, Mo, V, Cu, Ni are mass fractions of elements in steel.
2. An indicator of the position of the cast iron composition in relation to the eutectic point, characterizing its degree of graphitization, structure and properties - the carbon equivalent is determined by the equation:
C e \u003d C + 0.3 (Si + P),
where C, Si, P - mass fractions of elements in cast iron. At C e 4.26 - hypereutectic;
See also:
- Equivalent
- Nickel and chromium equivalent
- electrochemical equivalent
- chromium equivalent
- thermal equivalent of work
- nickel equivalent
- mechanical equivalent of heat
- chemical equivalent of the element
- - , a sequence of thermonuclear reactions in stars leading to the conversion of hydrogen into helium using carbon as a catalyst...
Physical Encyclopedia
- - an object or quantity of equal value, equivalent, which can serve as an expression or replacement for each other; accordingly equivalent - equivalent, equivalence - equivalence...
The Beginnings of Modern Natural Science
- - 1...
- - an item or quantity of equal value, equivalent or corresponding in any respect to others and which can serve either as an expression or as a substitute for: See also: - nickel and chromium - electrochemical equivalent...
Encyclopedic Dictionary of Metallurgy
- - Carbon potential - The ability of an environment containing active carbon to change or maintain a given level of carbon in steel...
Dictionary of metallurgical terms
- - oil prospecting criterion proposed by White, characterizing the dependence of oil content exc. on the degree of metamorphism of the org contained in them. substances...
Geological encyclopedia
- - ...
- - ...
merged. Apart. Through a hyphen. Dictionary-reference book
- - CARBON, -a, m. Chemical element, the most important component of all organic substances...
Explanatory dictionary of Ozhegov
- - carbon adj. 1. ratio with noun carbon associated with it 2. Characteristic of carbon, characteristic of it. 3. Consisting of carbon. 4. Containing carbon...
Explanatory Dictionary by Efremova
- - ...
- - ...
Spelling dictionary-reference book
- - chamois "histo-coal" ...
- - carbon "...
Russian spelling dictionary
- - ...
Word forms
- - ...
Synonym dictionary
"carbon equivalent" in books
author Kolesnik Yu. A.7.4. Carbon metabolism in the biosphere
From the book Current State of the Biosphere and Environmental Policy author Kolesnik Yu. A.7.4. Carbon metabolism in the biosphere Around the globe, according to scientists (Larcher, 1978, p. 128), plants annually sequester about 155,109 tons of carbon. Of this amount, land accounts for 61%, and the hydrosphere - 39% of its total amount. Very high primary productivity at
WHAT IS MR MINISTER MENTOR'S CARBON FOOTPRINT?
From the book Harsh Truths to Move Singapore Forward (excerpts from 16 interviews) by Lee Kuan YewWHAT IS MR MINISTER MENTOR'S CARBON FOOTPRINT? - Take your lifestyle. How environmentally responsible are you? Are you trying to reduce your carbon footprint? Do you sleep with the air conditioner on? - I sleep, but what can you do about it? Without
TNT equivalent
From the book Great Encyclopedia of Technology author Team of authorsTNT equivalent TNT equivalent is a characteristic of the explosion power of an atomic bomb and also its caliber. The TNT equivalent of an atomic bomb is the weight of a TNT charge, the explosion energy of which is equal to the explosion energy of a given nuclear weapon. TNT
Currency equivalent
From the book The Secrets of Gemstones author Startsev Ruslan VladimirovichCurrency equivalent In addition to all of the above, precious stones, including sapphire, play an important role as a currency equivalent. This is because they are often of great value. However, as experience has shown, their value remains
What is the carbon cycle?
From the book Everything about everything. Volume 2 author Likum ArkadyWeldability
Weldability of steel:
Structural grade steel 30G
Rice. 11. Form of penetration
Rice. 12. Structural components



Rice. 15. Crack resistance
Conclusion
Having considered three calculations for the weldability of 30G steel operating under the same conditions after each welding method. We can conclude that the best welding method for this steel is welding in a shielding gas environment (CO 2) with post-weld heating at 400 0 C for 2 hours. With this welding, we get the best structure of the weld metal, welding voltage, and the lowest probability of formation cracks.
Conclusion
In this course project, an indirect method for assessing the weldability of metal was considered through the “Weldability of Alloy Steels” program. It was also concluded that for 30G steel the best welding method is welding in a shielded gas environment (CO 2).
List of literature used in the implementation
Course project
1. Theory of welding processes: Textbook for universities specializing in “Equipment and technology of welding production” / V.N. Volchenko, V.M. Yampolsky, V.A. Vinokurov and others; Edited by V.V. Frolova. M.: Higher School, 1988. 559 p.
2. Welding in mechanical engineering: a Handbook. In 4 volumes. / Editorial Board: G.A. Nikolaev (prev.) and others. M: Mechanical Engineering, 1978-79.
3. Grader of steels and alloys / /V.G. Sorokin, A.V. Volosnikova, S.A. Vyatkin and others; Under the general editorship of B.G. Sorokina. M.: Mechanical Engineering, 1989. 640 p.
Weldability
Weldability is the property or combination of properties of metals to form, with the established welding technology, a permanent connection that meets the requirements determined by the design and operation of the product.
There are physical and technological weldability.
Physical weldability is the property of materials to produce a monolithic compound with a chemical bond. Almost all technical alloys and pure metals, as well as a number of combinations of metals and non-metals, have such weldability.
Technological weldability is a technological characteristic of a metal that determines its response to the effects of welding and its ability to form a welded joint with specified performance properties.
The weldability of a metal depends on its chemical and physical properties, crystal lattice, degree of alloying, presence of impurities and other factors.
Let us name the main indicators of the weldability of metals and their alloys:
Oxidation during welding heating, depending on the chemical activity of the metal;
Sensitivity to the thermal effects of welding, which is characterized by the tendency of the metal to grain growth, structural and phase changes in the weld and heat-affected zone, changes in strength and plastic properties;
Resistance to hot cracking;
Resistance to cold cracking during welding;
Sensitivity to pore formation;
Compliance of the properties of the welded joint with the specified operational requirements.
In addition to the listed main indicators of weldability, there are also indicators on which the quality of welded joints depends. These include the quality of weld formation, the magnitude of intrinsic stresses, the magnitude of deformations and warping of the materials and products being welded.
The oxidation of metal during welding is determined by the chemical properties of the material being welded. The more chemically active the metal, the greater its tendency to oxidize and the higher the quality of protection during welding should be. This is especially clearly seen in the example of iron-carbon alloys. As you know, steel mainly consists of iron with a constant admixture of carbon.
The weldability of steel is usually assessed by the following indicators:
The tendency of the weld metal to form hot and cold cracks;
Tendency to change the structure in the heat-affected zone and to form hardening structures;
Physical and mechanical qualities of the welding joint;
Compliance of the special properties of the welded joint with the technical conditions.
Welding technology (type of welding, welding materials, welding technique) is selected depending on the main indicator of weldability (or combinations of several indicators) for each specific material.
Based on carbon content, steels are divided into: low-carbon (up to 0.25% C); medium carbon (0.25-0.4% C); high-carbon (0.46-0.9% C). Low-carbon steels, widely used for building structures, are well welded. Welding of medium-carbon steels is possible subject to special technology, which usually includes preheating and subsequent heat treatment, eliminating hardening of the joint. Manual arc welding of high-carbon steels is not recommended. It is possible only if technology is followed, which, however, does not always provide a connection equal to the strength of the base metal.
In addition to carbon, the steel and weld contain Mn and Si, which enter the metal during the deoxidation process. To increase the strength characteristics and acquire special properties of steel (corrosion resistance, heat resistance, etc.), alloying of the metal with various useful elements is used, which, while improving its properties, at the same time worsen its weldability. Alloy steels are divided depending on the content of alloying elements into: low alloy (no more than 2.5%); alloyed (2.5-10%) and highly alloyed (more than 10%). The weldability of steel can be approximately determined by the number of alloying elements equivalent to carbon, using the formula:
Ce = C+Mn/6+Si/24+Cr/5+Ni/10+Mo/4+V/5+Cu/13+P/2 ,
where Ce is the carbon equivalent, %;
C, Mn, Si, etc. - the content of these elements in steel, %.
Weldability of steel 30G - EtoSteel structural alloyed. This type of steel is used for parts to be improved that require low strength: rods, axles, cylinders, disks, bolts, nuts, screws and others. Structural alloyed steels of type 30G are supplied in the form of rolled products in accordance with GOST 4543-71, GOST 2591-88, GOST 2879-88. At the beginning of the stamp there is a two-digit number indicating the carbon content in hundredths of a percent. The alloying elements are listed below. The number following the symbol of the element shows its content as a percentage. If the number does not stand, then the content of the element does not exceed 1.5%. To designate high-quality alloy steels, the symbol A is indicated at the end of the grade. For example, steel 30G (0.30%). It has high strength (σ in = 640...780 MPa, σ 0.2 = 440...540 MPa) and relatively low ductility (δ = 6...20%, ψ = 45%). It can be used at a temperature of -80 ° C (wall thickness is not more than 100 mm).
Steel 30G is welded to a limited extent. Methods of welding RDS, ADS submerged arc and gas shielding, EShS. We recommend heating and subsequent heat treatment. CTS without restrictions.
Weldability of steel:
Structural grade steel 30G limited welding. With an increase in carbon in the steel, the heat-affected zone and the weld are hardened, hardness increases, and welded joints become more brittle and prone to cracking.
Satisfactory steels have a carbon content of 0.25 to 0.35%. They are less prone to cracking and, with the correct welding conditions, a high-quality seam is obtained. To improve the quality of welding, heating is often used.
Methods for calculating weldability
4. Epifanov, G. I. Solid State Physics: textbook. manual for universities. - M.: Higher School, 1965. - 276 p.
5. Aleshin, N. P. Ultrasonic flaw detection: reference book. allowance / N. P. Aleshin, V. G. Lupachev. - Mn. : Higher School, 1987. - 271 p.
6. Ermolov, I. N. Theory and practice of ultrasonic testing / I. N. Ermolov. - M.: Mechanical Engineering, 1981. - 240 p.
LOMOVA Olga Stanislavovna, Candidate of Technical Sciences, Associate Professor (Russia), Associate Professor of the Department of Petrochemical Technologies and Equipment.
Correspondence address: 190567@ mail.ru MORGUNOV Anatoly Pavlovich, Doctor of Technical Sciences, Professor (Russia), Head of the Department of Mechanical Engineering Technology.
Address for correspondence: 644050, Omsk, Mira Ave., 11, TM department.
The article was received by the editor on February 25, 2015. © O. S. Lomova, A. P. Morgunov
UDC 621.791.011+669.14.018
B. E. LOPAEV R. R. KHISMATULIN I. I. KAGARMANOV A. M. USTYAN
Omsk State Technical University
EVALUATION OF WELDABILITY OF STEELS OF DIFFERENT CLASSES BY THE METHOD OF THE CHEMICAL EQUIVALENT OF CARBON_
Based on the calculation of the chemical equivalent of carbon, the susceptibility of carbon and alloy steels to the formation of cold cracks, related to the concept of “weldability of materials,” was assessed.
Key words: chemical equivalent of carbon, weldability, cold cracks, martensite, local concentration, incubation period.
The ability of materials to form a welded joint is determined by tests for weldability.
Weldability (connectability) is the property of a material to form a permanent connection with the required quality and level of physical, mechanical and functional properties of the connection both during its production and during operation of the product.
The main features characterizing the weldability of steels are the tendency to form cracks of various types and the mechanical properties of the welded joint.
According to weldability, steels are divided into four groups: first - well-welded; the second - satisfactorily welded; the third - limitedly welded; fourth - poorly welded steels.
The first group includes steels that can be welded using conventional technology, i.e. without heating before welding and during welding and without subsequent heat treatment. However, the use of heat treatment to relieve internal stresses is not excluded.
The second group includes steels that do not form cracks when welded under normal production conditions. This group also includes steels that require preheating, as well as preliminary and subsequent heat treatment, to prevent the formation of cracks.
The third group includes steels that are prone to crack formation under normal welding conditions. When welding, they are preliminarily subjected to heat treatment and heated. In addition, most steels in this group are heat treated after welding.
The fourth group includes steels that are most difficult to weld and prone to cracking. These steels are weldable to a limited extent, so welding is performed with mandatory preliminary heat treatment, with heating during the welding process and subsequent heat treatment.
When welding carbon and alloy steels, weldability is determined by cold cracking tests.
It is known that cold cracks arise in the metal of the heat-affected zone in the presence of three conditions: the formation of hardening microstructures (martensite); the presence of diffusion hydrogen and tensile stresses.
To assess the tendency of a metal to form cold cracks, the concept of the chemical equivalent of carbon is used. The mathematical approach to describing the chemical equivalent of carbon was based on the assumption that weldability can be determined by an indicator that determines what minimum critical cooling time is necessary for 100% martensite to form in the weld metal. The less prepared
Chemical composition of the investigated steels, %
Steel grade C B! Mn No. Cr Mo Si
low carbon
Steel St 3 sp 0.14-0.22 0.12-0.30 0.40-0.65 0.30 0.30 - 0.25
Steel 20 0.17-0.24 0.17-0.37 0.35-0.65 0.30<0,30 - 0,25
Steel 20g 0.17-0.24 0.17-0.37 0.70-1.00 0.25<0,25 - -
Steel 15 0.12-0.19 0.17-0.37 0.35-0.65 0.30 0.30 - 0.30
medium carbon
Steel St 4 sp 0.18-0.27 0.12-0.30 0.40-0.70 - - - -
Steel St 5 sp 0.28-0.37 0.15-0.35 0.50-0.80 - - - -
Steel 25 0.22-0.30 0.17-0.37 0.50-0.80 -<0,25 - -
Steel 40 0.37-0.45 0.17-0.37 0.50-0.81 -<0,25 - -
low-alloyed
15HSND 0.12-0.18 0.40-0.70 0.40-0.70 0.3-0.6 0.6-0.9 - 0.20-0.4
10G2S1 £0.12 0.90-1.20 1.30-1.65 £0.30 £0.30 - £0.30
20ХМ 0.15-0.25 0.17-0.37 0.40-0.70 - 0.8-1.1 0.40-0.60 -
10G2B £0.12 0.17-0.37 1.20-1.60 £0.30 £0.30 - £0.30
17GS 0.14-0.20 0.40-0.60 1.0- 1.40 £0.30 £0.30 - £0.30
16G2AF 0.12-0.18 0.17-0.37 1.30-1.70 - - - -
medium alloyed
12X5MA 0.15 0.6 0.5 - 4.0-6.0 0.5-0.6 -
20Х2МА 0.18-0.24 0.17-0.37 0.30-0.70 0.3-0.7 2.1-2.4 0.25-0.35 -
30HN2MFA 0.26-0.33 0.17-0.37 0.30-0.60 2.0-2.5 0.6-0.9 0.20-0.30 -
06NZ 0.04-0.08 0.3 0.5 3.0-4.0 - - -
20KhGSA 0.17-0.23 0.90-1.20 0.80- 1.10 - 0.8-1.1 - -
30KhGSNA 0.27-0.34 0.90-1.20 1.00-1.30 1.4-1.8 0.9-1.2 - -
significant time is required for the formation of a 100% martensitic structure (i.e., the higher the critical cooling rate), the better the weldability and the higher the resistance to cold cracking. This indicates that the preparatory processes associated with the formation of cold cracks are of a diffusion nature and are directly related to the redistribution of hydrogen in the weld metal. In the case of a short incubation period (1–10 s) for martensite formation, hydrogen is quickly fixed in the weld metal, but its local concentration is not sufficient to initiate the formation of cold cracks. In the case of a long incubation period for the formation of martensite (1000 - 2000 s), the time is quite enough for the embrittlement of the metal being welded as a result of the action of hydrogen. With a short incubation period, but subsequent long exposure, a gradual redistribution of hydrogen is possible, which causes the effect of delayed destruction.
The equation for the chemical equivalent of carbon is:
CE m = C+--+--Mn+-No +
Cr+--Mo+-Cu,
where C, Mn, etc. elements, %.
chemical concentration
The assessment of the hardenability of the HAZ metal is calculated using the equation:
1n(Mm) = A CEM + B,
AM - critical cooling time from temperature from 800 to 500 °C, s.
At CEM from 0.2 to 0.45%, steel has good weldability; at
SE m = 0.46 - 0.576% - satisfactory; at CE m = 0.577-0.782% - limited and at CE m = 0.783-1.0% - poor weldability.
The purpose of this work is to determine the weldability by the chemical equivalent of carbon of some low- and medium-carbon, low- and medium-alloy steels, the chemical composition of which is given in Table. 1.
The calculations of CEm and In(A^) are given below, and the graphical dependences of In(A^) on CEm are shown in Fig. 1. 1-4.
Calculation of CM and In(A^) using equations (1) and (2)
Low carbon steels
Steel St. 3 sp _ _ 0.12 0.40 0.30 0.30 0.25
38 6.0 12 1.8 9.1 1p(Ay = 11.26-0.427-3.51 = 1.29
for well-welded steels: St. 3 bd, 20, 20G, 15, St. 4 bd, 25, 06N3
Rice. 2. Influence of CEM on In(Ay) for satisfactorily welded steels: St. 5 sp, 15KhSND, 10G2S1, 10G2B, 17GS, 16G2AF
Rice. 3. Effect of CEM on 1p(A(m) for limited weldability steels: 40, 20ХГСА
se m = 0.17+017+035+030+025+025=0.422, %;
M 38 6.0 12 1.8 9.1
1p(Du = 11.26.0.42 -3.51 = 1.24
SE m = 0.17 + 0I + 035 + 030 + 025 + 025 = 0.422,%;
M 38 6.0 12 1.8 9.1
1p(Dn = 11.26.0.442 -3.51 = 1.46
^ 0.17 0.35 0.30 0.30 0.30 p lpg 0.
CEM = 0.12+--+--+--+--+--=0.406, %; M 38 6.0 12 1.8 9.1
1p(Dn = 11.26.0.406 -3.51 = 1.06
All of the above low-carbon steels have the chemical equivalent of carbon CEM<0,45, поэтому они относятся к хорошо сваривающимся сталям.
Rice. 4. Effect of CEM on 1p(A(m) for poorly weldable steels: 20KhM, 12Kh5MA, 20Kh2MA, 30KhN2MFA, 30KhGSNA
Medium carbon steels Steel St. 4 sp
SE m =0.27+030+070=0.394, %; M 38 6.0
1p(DM) = 11.26.0.394 -3.51= 0.92
Assessment of weldability of steels
Steel grade CEM, % 1p(LGm) LGm, s Weldability
low carbon
Steel St 3 sp 0.427 1.29 3.661 good
Steel 20 0.422 1.24 3.459 good
Steel 20g 0.442 1.46 4.331 good
Steel 15 0.406 1.06 2.889 good
medium carbon
Steel St 4 sp 0.394 0.92 2.524 good
Steel St 5 sp 0.492 2.02 7.606 satisfactory
Steel 25 0.429 1.32 3.743 good
Steel 40 0.626 3.53 34.398 limited
low-alloyed
15HSND 0.575 2.96 19.375 satisfactory
10G2S1 0.564 2.84 17.115 satisfactory
20ХМ 0.869 6.27 531.126 bad
10G2B 0.529 2.44 11.542 satisfactory
17GS 0.541 2.58 13.210 satisfactory
16G2AF 0.464 1.71 5.551 satisfactory
medium alloyed
12Х5МА 3.842 39.75 1.833 1017 bad
20Х2МА 1.534 11.98 160011.345 bad
30ХН2МФА 0.899 6.61 743.969 bad
06NZ 0.402 1.01 2.762 good
20ХГСА 0.771 5.17 176.09 limited
30KhGSNA 1.076 8.42 4536.90 bad
Steel St. 5 sp
CEM = 0.35 +035+080 = 0.492, %; m 38 6.0
1p(LM) = 11.26-0.492 -3.51= 2.02
Steel 10G2S1
™ psh 0.9 1.3 0.30 0.30 0.30%
CEM = 0.10+--+--+--+--=0.564, % m 38 6.0 12 1.8 9.1
1p (LM) \u003d 11.26-0.564 -3.51 \u003d 2.84
SE m = 0.22 +
Steel 25 0.17 0.50 0.22
1p(Lu = 11.26-0.429 -3.51 = 1.32
Steel 20ХМ
P10 0.17 0.40 0.8 0.40 p ogp 0. SEm = 0.18+--+--+--+--=0.869, %; m 38 6.0 1.8 2.3
1p(Lu = 11.26-0.869 -3.51= 6.27
0,17 0,50 0,25 --+-+-
1p(Lu = 11.26-0.626 -3.51= 3.53
For steels Art. 4 and 25 chemical equivalent of carbon CEM<0,45 %, и они относятся к хорошо сваривающимся сталям. У стали 40 СЕм = 0,626 %, поэтому ее можно отнести к ограниченно сваривающимся, сталь Сп. 5 СЕм = 0,492 %, поэтому она относится удовлетворительно сваривающимся сталям.
Low alloy steels
SE m = 0.12 +
Steel 15HSND 0.40 0.40 0.30 0.60 0.20
38 6.0 12 1.8 9.1 1p(Lu = 11.26-0.57 -3.51= 2.96
Steel 10G2B
0,17 1,2 0,30 0,30 0,30 „ 10 +--+--+--+--=0 38 6,0 12 1,8 9,1
1p(Lu = 11.26-0.529-3.51=2.44
Steel 17GS 0.40 1.0 0.30 0.30 0.30
-+-■+--+--+--=0,541, %
38 6.0 12 1.8 9.1 1p(Lu = 11.26-0.541 -3.51= 2.58
Steel 16G2AF
SE m = 0.18 + 037+165 = 0.464, %; m 38 6.0
1p (Lu \u003d ACEm + B \u003d 11.26-0.464-3.51 \u003d 1.71
Steels 10G2S1, 10G2B, 17GS, 15KhSD, 16G2AF are classified as satisfactorily weldable steels, 20KhM are classified as poorly weldable steels.
Medium alloy steels
Steel 12Х5МА
^plg 0.6 0.5 6 0.6 „, CEM = 0.15+-++-++-+-=3.842, % M 38 6.0 1.8 2.3
ln(AfM) = 11.26-3.842 -3.51= 39.75
Steel 20Х2М2
0,17 0,3 0,3 2,1 0,25 „ --+-++-++-+--=1,534, % 38 6,0 12 1,8 2,3
ln(AfM) = 11.26-1.534-3.51 = 11.98
Steel 30KhN2MFA 0.17 0.3 2.0 0.6 0.20
CE M = 0.26 +--+-++-++-++■
38 6.0 12 1.8 2.3 ln(AiM) = 11.26-0.899 -3.51= 6.6
Steel 06NZ
030 + 050 + M=0 02, %; 38 6.0 12
ln(AiM) = 11.26-0.402-3.51 = 1.01
step to build graphs corresponding to satisfactory, limited and poor weldability (Fig. 2 - 4).
From the table It can be seen from Table 2 that the shorter the critical cooling time for 100% martensite, the lower the value of the chemical equivalent of carbon, the higher the weldability and the lower the probability of cold cracking in carbon and alloy steels.
With a small value of time (1 - 10) s, the local concentration of hydrogen is insufficient for the formation of cold cracks.
The numerical value of the time that affects the weldability of steels (Table 2) can be distributed as follows: (1-5) s - good; (5-18) s - satisfactory; at AtM>18 s - limited and poor weldability.
Thus, the information presented in the article will be useful to developers of materials to be welded, technologists when designing welding technology for various structures, and students when studying the discipline “Theory of Welding Processes”.
Bibliography
Steel 20HGSA
CE« = 0.17 + 09+08+08 = 0.771, %;
1p(Dn = 11.26-0.771-3.51=5.1
Steel 30HGSNA
n ^ 0.9 1.0 1.4 0.9 1 ppg 0.
CEM = 0.27 I-1-I-:-I-:-I--=1.076, %; m 38 6.0 12 1.8
1p(Dn = 11.26-1.076 -3.51=8.4
Steel 06N3 has CEm = 0.402, it belongs to well-welded steels. Steel 20KhGSA has CEm = 0.771, so it belongs to limitedly weldable steels. Steels 12Kh5MA, 20Kh2M2, 30KhN2MFA, 30KhSNA belong to poorly welded steels.
Obtained as a result of the calculation of CEm and Ip(Dn, we summarize in Table 2.
Let us construct graphical dependences of the chemical equivalent of carbon on the logarithm of the critical cooling time of 100% martensite by weldability group.
For example, to build a graph of "good weldability" it is necessary from Table. 2 select CEM values in the range of 0.2 - 0.45% and the corresponding values of 1p(Du. In the same way you need to
1. Yushchenko, K. A. Weldability and promising processes of welding materials [Text] / K. A. Yushchenko // Automatic welding. - 2004. - No. 9. - P. 40 - 45.
2. Handbook of the welder / Ed. V.V. Stepanova. - 3rd ed. - M. : Mashinostroenie, 1974. - 520 p.
3. Kostin, V. A. Mathematical description of carbon equivalent as a criterion for assessing the weldability of steels [Text] / V. A. Kostin // Automatic welding. - 2012. - No. 8. - P. 12-17.
4. Technology of electric welding of metal and alloys by fusion [Text] / Ed. B. E. Paton. - M. : Mashinostroenie, 1974. - 768 p.
LOPAEV Boris Evgenievich, Candidate of Technical Sciences, Associate Professor (Russia), Associate Professor of the Department of Mechanical Engineering and Materials Science.
Khismatulin Roman Rafikovich, student gr. S-510
KAGARMANOV Igor Igorevich, student gr. SM-312
engineering institute.
USTYAN Armen Manvelovich, undergraduate gr. SPM-
514 Engineering Institute.
Correspondence address: 644050, Omsk, Mira Ave., 11.
The article was received by the editor on February 26, 2015. © B-E-Lopaev, R. R. Khismatulin, I-I-Kagarmanov, A-M-Ustyan
Bookshelf
Mylov, G-V- Methodological foundations of automation of design and technological design of flexible multilayer printed circuit boards / G-V- Mylov, A-I- Taganov- - M-: Hotline-Telecom, 2014- - 167 c- - ISBN 978-5 -9912-0367-8-
The methodological foundations are outlined, including the modern concept of constructing information support for the stages of the life cycle of flexible multilayer printed circuit boards (FMC), the basis for the analysis and synthesis of design design and technological solutions and information support for the stages of computer-aided design and technological preparation for the production of FMC products. For specialists, it will be useful for graduate students and students.
Welding is one of the methods for creating one-piece metal structures. The strength of the seam formed at the junction of the component parts depends on such a characteristic of steel as “weldability”.
Classification of steel according to the degree of its weldability
Steel is represented by various groups of grades, each with its own physical and chemical properties. As a result, metal products have different weldability rates. Depending on this parameter, iron-carbon alloys are divided into four categories.
- good
When welding, a high-quality seam is obtained. The metal does not require preheating to carry out the work, and the work itself is carried out in the usual way and using all known technologies. - Satisfactory
To create a high-quality welded joint, steel products must be prepared, that is, heated. - Limited
Before welding, metal products are first heated, and after joining they are also subjected to heat treatment. - bad
Such steel is characterized by the fact that during welding (after it) cracks form on the surface, and “hardening” structures can also appear, reducing the strength and reliability of the joint, making it brittle.
Methods for calculating the carbon equivalent
The properties of steel generally depend on the presence of other metals in the alloy of iron and carbon. Knowing their content, using an empirical formula it is not difficult to calculate the value of the so-called carbon equivalent (Ce). This value allows you to determine what results to expect from welding metal products.
In Russia, to evaluate the welding characteristics of rolled products used to create structures, they use the formula approved by GOST GOST 27772-88:
Ce=C+(P/2)+(Cr/5)+(Mn/6)+(Cu/13)+(V/14)+(Si/24)+(Ni/40).
In Europe, the following relationship is used for calculations:
Se=C+(Mn/6)+(Cr+Mo+V)/5 + (Ni+Cu)/15.
In Japan, this method for determining the carbon equivalent:
Ce=C+(Mo/4)+(Cr/5)+(Mn/6)+(Si/24)+(Ni/40),
where C, P, Cr, Mn, Cu, V, Si, Ni, Mo are the mass fractions (in %) of carbon, phosphorus, chromium, manganese, copper, vanadium, silicon, nickel, molybdenum.
Steel is considered not prone to cracking if the carbon equivalent value “C” is less than 0.45%. Otherwise, when there is already a possibility of their occurrence, the parts requiring connection must be heated before welding.
Calculation of the hardness value in the heat-affected zone
The next parameter to pay attention to is the hardness of the heat-affected zone (HAZ). This is the name of the section of the product, which is located near the formed seam. In this region, under the influence of temperature, phase transformations occur with a change in the internal structure of the metal. Sometimes this is fraught with the fact that the steel becomes brittle.
The hardness of the metal in this zone is determined by the Vickers method. If its values are in the range of 350-400 on a special HV scale, then in the HAZ area there are definitely decomposition products of austenite (one of the modifications of iron and its alloys), which initiate the formation of cold cracks.
The maximum hardness value of carbon and low-alloy steel is calculated using data on the chemical composition of the metal using this formula:
HVmax = 90+1050*C+75*Mn+47*Si+31*Cr+30*Ni,
where C, Mn, Si, Cr, Ni are mass fractions (in percent) of chemical elements.
Determination of the sensitivity of steel to the formation of cold cracks
Cold cracks form after welding due to tensile residual stresses. Their strength depends on the rigidity of the resulting structure and the thickness of the seam. Its value can be determined by the stiffness intensity coefficient - K. It characterizes the applied force, which opens the gap by 1 mm, which is also 1 mm wide in the welded joint. It is calculated like this:
where Kq is a constant, which is considered to be equal to 69, S is the thickness of the steel sheet (in mm). It is important to note that the ratio is only valid if the sheet thickness does not exceed 150 mm.
How steel can be susceptible to cold cracking can be determined by the parametric equation:
Pw=Рш+(Н/60)+0.25*К/105,
where Рш is the “embrittlement” coefficient (this is the name for the process when a metal goes from a viscous state to a brittle one), H is the amount of diffusion hydrogen, K is the hardness intensity coefficient.
The value of Psh is found by solving the Bes-Sio equation:
Psi=C+5*B+Si/30+ Ni/60+(Mo+V)/15+(Mn+Cu+Cr)/20.
The results of repeated studies helped to establish the threshold value at which the sensitivity of steel to the formation of cold cracks manifests itself. This happens if the Pw value is greater than 0.286.
Ways to eliminate cold cracks during welding
The formation of cracks deteriorates the metal surface and, accordingly, reduces the strength of the finished structure. The following will help prevent them:
- revision (change) of design solutions, which will reduce rigidity in the area of the welded joint;
- careful monitoring of the progress of welding under optimal conditions will help reduce the content of diffusion hydrogen;
- carrying out welding work in compliance with special parameters that will prevent embrittlement of the metal and will facilitate the removal of diffusion hydrogen from the seam.
Of the listed methods for reducing the likelihood of cold cracks occurring during welding, the most popular is the last one.