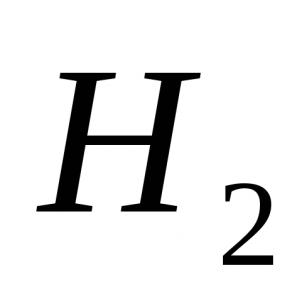Test “Nitrogen and its compounds. Topic: Nitrogen and its compounds. (Testing) test in chemistry (grade 9) on the topic Test on the topic of nitrogen and its compounds
Test "Nitrogen and its compounds"
Option 1 1. The strongest molecule a) H 2; b) F 2 ; c) O 2; d) N 2 . 2. Phenolphthalein color in ammonia solution: a) crimson; b) green; c) yellow; d) blue. 3. The oxidation state is +3 at the nitrogen atom in the compound: a) NH 4 NO 3; b) NaNO 3 ; c) NO 2; d) KNO 2. 4. During thermal decomposition of copper (II) nitrate, the following are formed:a) copper (II) nitrite and O 2 ;b) nitric oxide(IV) and O 2 ;c) copper(II) oxide, brown gas NO 2 and O 2 ; d) copper (II) hydroxide, N 2 and O 2. 5. Which ion is formed by the donor-acceptor mechanism? a) NH 4 + ; b) NO 3 - ; c) Cl - ; d) SO 4 2–. 6. Specify strong electrolytes: a) nitric acid; b) nitrous acid; c) an aqueous solution of ammonia; d) ammonium nitrate. 7. Hydrogen is released during the interaction: a) Zn + HNO 3 (razb.); b) Cu + HCl (solution); c) Al + NaOH + H 2 O; d) Zn + H 2 SO 4 (razb.); e) Fe + HNO 3 (conc.). 8. Write an equation for the reaction of zinc with very dilute nitric acid if one of the reaction products is ammonium nitrate. Specify the coefficient in front of the oxidizing agent. 9.Name the substances A, B, C. Option 2 1. It is impossible to collect by the method of displacement of water: a) nitrogen; b) hydrogen; c) oxygen; d) ammonia. 2. The reagent for the ammonium ion is a solution of: a) potassium sulfate; b) silver nitrate; c) sodium hydroxide; d) barium chloride. 3. When interacting with HNO 3 (conc.) gas is formed with copper shavings: a) N 2 O; b) NH 3; c) NO 2 ; d) H 2 . 4. Thermal decomposition of sodium nitrate produces: a) sodium oxide, brown gas NO 2, O 2; b) sodium nitrite and O 2; c) sodium, brown gas NO 2, O 2; d) sodium hydroxide, N 2, O 2. 5. The degree of nitrogen oxidation in ammonium sulfate: a) -3; b) -1; c) +1; d) +3. 6. With which of the following substances does concentrated HNO react? 3 under normal conditions? a) NaOH; b) AgCl; c) Al; d) Fe; e) Cu. 7. Specify the number of ions in the abbreviated ionic equation for the interaction of sodium sulfate and silver nitrate: a) 1; b) 2; in 3; d) 4. 8. Write an equation for the interaction of magnesium with dilute nitric acid if one of the reaction products is a simple substance. Specify the coefficient in the equation in front of the oxidizing agent. 9. Write reaction equations for the following transformations:
Name the substances A, B, C, D.
Answers
Option 1 1 - G; 2 - a; 3 - G; 4 - in; 5 - a; 6 - a, d; 7 - c, d; 8 – 10,


2Ag + + SO 4 2– = Ag 2 SO 4;
8 – 12, 9. A - NO, B - NO 2, C - HNO 3, D - NH 4 NO 3,
Option 1.
Chemical sign of nitrogen: a) P b) N c) As d) Ne
A nitrogen molecule contains: a) a double bond b) a single bond c) a triple bond
Molecular formula of nitrogen: a) N b) N 2 c) N 3 d) Ne
On the physical properties of nitrogen not applicable : a) gas b) odorless c) poorly soluble in water d) brown.
Nitrogen in compounds cannot show oxidation state a) +1 b) -3 c) +6 d) +5
Nitrogen does not react with a) metals b) phosphorus c) hydrogen d) oxygen
When nitrogen reacts with hydrogen, a) phosphine is formed b) nitric oxide (II) c) methane d) ammonia
When nitrogen reacts with oxygen, a) nitric oxide (II) is formed b) nitric oxide (I) c) nitric oxide (IV) d) nitric oxide (V)
Compounds of nitrogen with metals are called a) nitrites b) nitrides c) nitrates d) ammoniates.
The oxidation state of nitrogen in ammonia a) -3 b) +5 c) +3 d) +2
Which of the properties not applicable to ammonia: a) gas c) poorly soluble in water c) has a pungent odor d) easily liquefies under pressure
Ammonia solution has a medium: a) acidic b) alkaline c) neutral d) slightly acidic
Ammonia does not interact a) with acids b) with alkalis c) with water d) with oxygen
When ammonia is burned, a) nitric oxide (II) is formed b) water c) nitrogen d) nitric oxide (IV)
During the catalytic oxidation of ammonia, in addition to water, a) nitric oxide (II) is formed b) nitric oxide (IV) c) nitric oxide (III) d) nitric oxide (V)
When ammonia reacts with hydrochloric acid, a) ammonia chloride b) ammonium chloride c) ammonium sulfate d) ammonium nitrate is formed
Ammonia can be obtained in the laboratory by reacting a) ammonium salt with alkali b) ammonium salt with acid c) hydrogen with nitrogen d) ammonium salt with other salts.
Ammonia is produced in industry a) ammonium salts with alkali b) ammonium salts with acid c) hydrogen with nitrogen d) ammonium salts with other salts.
can not form salts a) acidic b) medium c) basic
Ammonium salts cannot be obtained a) by the interaction of ammonia with acids b) by the interaction of an ammonia solution with acids c) by the interaction of ammonium salts with acids d) by the interaction of ammonium salts with alkali.
What reaction is qualitative for ammonium salts a) interaction with alkali b) interaction with acids c) interaction with other salts d) interaction with water.
A sign of a qualitative reaction to the ammonium ion is a) the evolution of a gas that causes turbidity of lime water b) the evolution of gas with the smell of ammonia c) the evolution of a white precipitate d) the evolution of brown gas.
When ammonium salts are heated, a) water is released b) decomposition with the release of gases c) evaporation d) a change in their color.
The decomposition of ammonium nitrate produces: a) nitrogen and water b) nitric oxide (I) and water c) nitric oxide (II) and water d) nitric oxide (IV) and water.
Nitrogen does not form oxide with the formula a) NO 2 b) NO 3 c) NO d) N 2 O
Acidic oxide is not a) NO 2 b) N 2 O 3 c) N 2 O 5 d) NO
Non-salt-forming oxides include a) NO 2 b) N 2 O 3 c) N 2 O 5 d) N 2 O
Nitric acid does not include a) liquid b) monobasic c) strong oxidizing agent d) odorless
The oxidation state of nitrogen in nitric acid a) +3 b) +5 c) +1 d) -3
not formed
The interaction of nitric acid with metals does not form a) water b) hydrogen c) nitric oxide d) salt
When concentrated nitric acid interacts with metals in the voltage series after hydrogen, in addition to water and salt,
When dilute nitric acid reacts with copper, in addition to water and salt,
a) nitric oxide (II) b) nitric oxide (I) c) nitric oxide (IV) d) ammonia.
Nitric acid does not react with a) sulfur oxide (IV) b) sodium hydroxide c) barium oxide d) calcium carbonate.
Test on the topic "Nitrogen and its compounds."
Option 2.
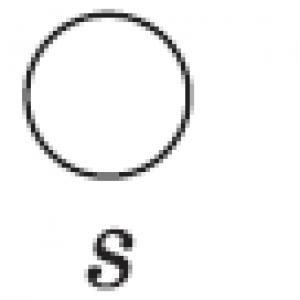
Chemical sign of nitrogen: a) N b) Nb c) Ar d) S
A nitrogen molecule contains: a) a hydrogen bond b) a covalent polar bond c) a covalent non-polar bond
Structural formula of nitrogen: a) N=N b) N≡ N c) N- N d) :N::: N:
On the physical properties of nitrogen not applicable : a) gas b) with a pungent odor c) poorly soluble in water d) heavier than air.
Nitrogen in the air by volume: a) 50% b) 65% c) 78% d) 85%
Nitrogen in compounds cannot show the oxidation state a) -1 b) +3 c) +4 d) +5
Nitrogen at room temperature reacts with a) oxygen b) lithium c) hydrogen d) sodium
When nitrogen reacts with hydrogen, a) hydrogen sulfide is formed b) nitrogen oxide (IV) c) ammonia d) nitrogen oxide (II)
The interaction of nitrogen with oxygen produces a) nitric oxide (II) b) nitric oxide (I) c) nitric oxide (IV) d) nitric oxide (III)
Compounds of nitrogen with metals are called a) nitrates b) nitrites c) nitrides d) ammonium salts.
The oxidation state of nitrogen in ammonia a) +3 b) +4 c) -3 d) +2
Which of the properties not applicable to ammonia: a) gas c) highly soluble in water c) odorless d) lighter than air
Ammonia solution has a medium: a) acidic b) neutral c) alkaline d) weakly alkaline
Ammonia does not interact a) with oxygen b) with hydrogen c) with water d) with acids
When burning ammonia formed a) nitric oxide (V) b) nitric oxide (I) c) nitrogen d) water
In the catalytic oxidation of ammonia, in addition to water, a) nitric oxide (V) is formed b) nitric oxide (III) c) nitric oxide (IV) d) nitric oxide (II)
When ammonia reacts with sulfuric acid, a) ammonia chloride b) ammonium chloride c) ammonium sulfate d) ammonium nitrate is formed
Ammonia can be obtained in the laboratory by reacting a) ammonium chloride with potassium hydroxide b) ammonium sulfate with hydrochloric acid c) hydrogen with nitrogen d) ammonium salts with others with acids.
Ammonia is produced in industry by the interaction of a) hydrogen with nitrogen b) ammonium chloride with alkali c) ammonium salt with acid d) ammonium salt with other salts.
When interacting with acids, ammonia can not form salts a) basic
b) medium c) sour
ammonium salts can't get a) the interaction of ammonium salts with alkali b) the interaction of ammonia solution with alkalis c) the interaction of ammonia solution with acids d) the interaction of ammonium salts with acids.
Ammonium salts can be recognized with the help of a) acid b) alkali c) another salt d) water.
A sign of a qualitative reaction to the ammonium ion is a) evolution of gas with the smell of ammonia; evolution of gas; b) evolution of a gas that causes turbidity of lime water; c) evolution of brown gas; d) evolution of a white precipitate.
When ammonium carbonate is heated not happening
a) release of water b) carbon dioxide c) release of ammonia d) release of nitric oxide (I).
The decomposition of ammonium nitrite produces:
a) nitrogen and water b) nitric oxide (I) and water c) nitric oxide (II) and water d) nitric oxide (IV) and water.
Nitrogen does not form oxide with the formula a) NO 2 b) N 2 O 5 c) NO d) N 2 O 7
It is not an acid oxide a) NO 2 b) N 2 O 3 c) N 2 O d) N 2 O 5
Non-salt-forming oxides include a) NO b) N 2 O 3 c) N 2 O 5 d) NO 2
Nitric acid does not include a) liquid b) dibasic c) strong oxidizing agent d) oxygen-containing
The oxidation state of nitrogen in nitric acid a) -3 b) +5 c) +4 d) +3
When nitric acid decomposes in the light formed a) water b) oxygen c) ammonia d) nitric oxide (IV).
When nitric acid reacts with metals, it does not form
a) nitrogen compound b) salt c) water d) hydrogen
In the interaction of concentrated nitric acid with silver, in addition to water and salt, a) nitric oxide (I) b) nitric oxide (IV) c) nitric oxide (II) d) ammonia is formed.
When dilute nitric acid reacts with silver, in addition to water and salt,
a) nitric oxide (II) b) nitric oxide (I) c) nitric oxide (IV) d) ammonia.
Nitric acid does not react with a) phosphorus (V) oxide b) sodium oxide c) barium hydroxide d) sodium carbonate.
Option number 1.
Task number 1
1. Has no smell.
2. Has an odor.
3. Burns in oxygen.
4. Interacts with acids to form salts.
5. In a molecule between atoms there is a covalent non-polar bond.
6. In a molecule between atoms there is a covalent polar bond.
7. Does not burn in the air.
8. Nitrogen oxidation state -3.
9. Slightly soluble in water.
10. The aqueous solution has an alkaline environment.
Task number 2
1. N 2 O 3 A) ammonium nitrate
2. NH 3 B) ammonium sulfate
3. NH 4 NO 3 B) nitric oxide (III)
4. NH 4 HSO 4 D) ammonium hydrosulfate
5. NO 2 D) nitric oxide (V)
E) nitric oxide (IV)
G) ammonia
Task number 3
N 2 NO NO 2 HNO 3 NO 2
Exercise№4
C + HNO 3 \u003d CO 2 + NO 2 + H 2 O
Task number 5
NH 4 NO 2 = N 2 O + H 2 O
.
4) Solid nitrates are thrown into the fire, and if there is no bright flash, it is nitrate.
Test on the topic "NITROGEN AND ITS COMPOUNDS".
Option number 2.
Task number 1
Distribute which of the properties belong to nitrogen, and which to ammonia? Record data with property numbers in a table.
1. Has a pungent odor
2. Highly soluble in water.
3. Does not interact with air.
4. In a molecule between atoms, the bond is covalent polar.
5. In a molecule between atoms, the bond is covalent non-polar.
6. Colorless and odorless gas.
7. Nitrogen oxidation state -3.
8. The aqueous solution has an alkaline environment.
9. Gives a reaction with oxygen during a thunderstorm.
10. Used in tubes for storing old paintings.
Task number 2
Match formulas and names of substances.
1. N 2 O 5 A) ammonium sulfate
2. NO B) ammonium carbonate
3. (NH 4) 2 SO 4 B) nitric oxide (II)
4. NH 4 H CO 3 D) ammonium bicarbonate
5.N 2 O D) nitric oxide (V)
E) nitric oxide (I)
G) ammonium hydrosulfate
Task number 3
Write reaction equations for the following transformations.
NO NO 2 HNO 3 NaNO 3 NaNO 2
Exercise№4
Arrange the coefficients using the electronic balance method, indicate the oxidizing agent and reducing agent.
S + HNO 3 \u003d H 2 SO 4 + NO 2 + H 2 O
Task number 5
Find 4 mistakes in this text, write down the mistakes. Then write it down correctly next to it.
1) When heated, nitrates decompose. The decomposition reaction of nitrates of different metals proceeds in the same way.
2) Only the process of thermal decomposition of ammonium nitrate proceeds in a peculiar way:
NH 4 NO 2 = N 2 O + H 2 O
3) Solutions of nitrates are determined using copper without heating.
4) Solid nitrates are thrown into the fire, and if there is no bright flash, it is nitrate.