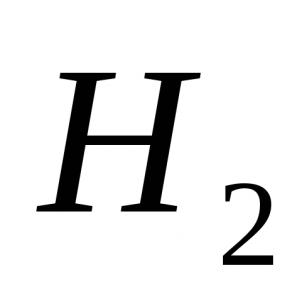Getting carbon. Chemical properties of saturated monobasic carboxylic acids
Carboxylic acids are derivatives of hydrocarbons containing one or more carboxyl groups.
The number of carboxyl groups characterizes the basicity of the acid.
Depending on the number of carboxyl groups, carboxylic acids are divided into monobasic carboxylic acids (containing one carboxyl group), dibasic (containing two carboxyl groups) and polybasic acids.
Depending on the type of radical associated with the carboxyl group, carboxylic acids are divided into saturated, unsaturated and aromatic. Limiting and unsaturated acids are combined under the general name of aliphatic or fatty acids.
Monobasic carboxylic acids
1.1 Homologous series and nomenclature
The homologous series of monobasic saturated carboxylic acids (sometimes called fatty acids) begins with formic acid
Homologous series formula
The IUPAC nomenclature permits the preservation of many acids by their trivial names, which usually indicate the natural source from which this or that acid was isolated, for example, formic, acetic, butyric, valeric, etc.
For more complex cases, the names of acids are derived from the name of hydrocarbons with the same number of carbon atoms as in the acid molecule, with the addition of the ending -ovaya and words acid. Formic acid H-COOH is called methanoic acid, acetic acid CH 3 -COOH is called ethanoic acid, etc.
Thus, acids are considered as derivatives of hydrocarbons, one link of which is converted into carboxyl:

When naming branched chain acids according to rational nomenclature, they are considered as derivatives of acetic acid, in the molecule of which hydrogen atoms are replaced by radicals, for example, trimethylacetic acid (CH 3) 3 C - COOH.
1.2 Physical properties of carboxylic acids
Only from purely formal positions can the carboxyl group be considered as a combination of carbonyl and hydroxyl functions. In fact, their mutual influence on each other is such that it completely changes their properties.
The polarization of the C=0 double bond, common for carbonyl, increases strongly due to the additional contraction of a free electron pair from the neighboring oxygen atom of the hydroxyl group:

The consequence of this is a significant weakening of the O-H bond in hydroxyl and the ease of splitting off a hydrogen atom from it in the form of a proton (H +). The appearance of a reduced electron density (δ+) on the central carbon atom of the carboxyl also leads to the contraction of the σ-electrons of the neighboring C-C bond to the carboxyl group and the appearance (as in aldehydes and ketones) of a reduced electron density (δ +) on the α-carbon atom of the acid .
All carboxylic acids are acidic (detected by indicators) and form salts with hydroxides, oxides and carbonates of metals and with active metals:
Carboxylic acids in most cases are dissociated in aqueous solution only to a small extent and are weak acids, significantly inferior to such acids as hydrochloric, nitric and sulfuric. So, when dissolving one mole in 16 liters of water, the degree of dissociation of formic acid is 0.06, acetic acid - 0.0167, while hydrochloric acid is almost completely dissociated with this dilution.
For most monobasic carboxylic acids RK a \u003d 4.8, only formic acid has a lower pKa value (about 3.7), which is explained by the absence of the electron-donating effect of alkyl groups.
In anhydrous mineral acids, carboxylic acids are protonated at oxygen to form carbocations:

The shift of electron density in the molecule of undissociated carboxylic acid, which was mentioned above, lowers the electron density on the hydroxyl oxygen atom and increases it on the carbonyl one. This shift is further increased in the anion of the acid:

The result of the shift is a complete equalization of the charges in the anion, which actually exists in the form A - the resonance of the carboxylate anion.
The first four representatives of the carboxylic acid series are mobile liquids, miscible with water in all respects. Acids, the molecule of which contains from five to nine carbon atoms (as well as isobutyric acid), are oily liquids, their solubility in water is low.
Higher acids (from C 10) are solids, practically insoluble in water; during distillation under normal conditions, they decompose.
Formic, acetic and propionic acids have a pungent odor; the middle members of the series have an unpleasant odor, the higher acids have no odor.

The physical properties of carboxylic acids are affected by a significant degree of association due to the formation of hydrogen bonds. Acids form strong hydrogen bonds, since the O-H bonds in them are highly polarized. In addition, carboxylic acids are able to form hydrogen bonds with the participation of the oxygen atom of the carbonyl dipole, which has a significant electronegativity. Indeed, in the solid and liquid state, carboxylic acids exist mainly in the form of cyclic dimers:
Such dimeric structures persist to some extent even in the gaseous state and in dilute solutions in non-polar solvents.
13.1.1. Hydrocarbon oxidation. There are two ways: the oxidation of lower alkanes C 4 -C 8 mainly to acetic acid and the oxidation of solid paraffin with the formation of synthetic fatty acids (FFA) with a straight chain of carbon atoms C 10 -C 20, which are raw materials for the synthesis of surfactants (surfactants) .
The process proceeds in the liquid phase when heated or in the presence of catalysts. During the oxidation of alkanes, destruction occurs along the bonds between secondary carbon atoms; therefore, acetic acid is mainly formed from n-butane, and methyl ethyl ketone and ethyl acetate are formed as by-products.
13.1.2 Syntheses based on carbon monoxide (II). Carboxylic acids are obtained from carbon monoxide by the carbonylation reaction:
Double bond addition in acid catalysis always proceeds according to Markovnikov's rule, as a result of which a straight-chain acid is obtained only from ethylene, and α-methyl-substituted acids from its homologues. This method is of particular interest for the synthesis of acids with a tertiary radical (non-acids) from branched olefins. (Koch reaction):

The reaction mechanism consists in the preliminary protonation of the alkene with an acid to form a carbenium ion, its interaction with CO to obtain acylium - cation and the reactions of the latter with water to form a carboxylic acid:
Neoacids and their salts have a very high solubility and viscosity, and their esters - stability to hydrolysis, which provides them with a wide application in a number of industries.
The carbonylation of alcohols is catalyzed by metal complexes (Ni, Co, Fe, Pd). The process has been implemented in industry for the synthesis of acetic acid from methanol and is characterized by high economic performance.
Acids are also obtained by oxidation of aldehydes (a product of oxosynthesis).
Laboratory methods for obtaining carboxylic acids
Oxidation of alkanes.
Alkene oxidation.
13.2.3. Oxidation of primary alcohols.
13.2.4. Oxidation of aldehydes and ketones. Aldehydes are oxidized much more easily than ketones. In addition, the oxidation of aldehydes leads to the formation of acids with the same number of carbon atoms, while the oxidation of ketones proceeds with the breaking of carbon-carbon bonds (two acids or an acid and a ketone are formed):

The oxidizers are potassium permanganate or dichromate. The oxidation of ketones requires more stringent conditions than those of aldehydes.
13.2.5. Hydrolysis of nitriles. Nitriles are obtained by the interaction of haloalkanes with potassium cyanide, hydrolysis is carried out with aqueous solutions of acids or alkalis. In an acidic environment, nitrogen is released in the form of an ammonium salt:

in alkaline - in the form of ammonium hydroxide, which decomposes with the release of ammonia, the acid is obtained in the form of a salt:

13.2.6. Grignard synthesis. When organomagnesium compounds interact with carbon dioxide, salts of carboxylic acids are formed:
A strong acid (usually HCl) converts the salt to an acid:
Hydrolysis of fats
Fats are esters of carboxylic acids and glycerol (triglycerides). The carboxylic acids that make up fats have a carbon chain from 3 to 18 carbon atoms.
Boiling fats or oils with aqueous solutions of alkalis (NaOH, KOH) leads to the formation of salts of carboxylic acids and glycerol.
This operation is called saponification, since salts of carboxylic acids are used to make soap.
Hydrolysis of derivatives of carboxylic acids.
Physical Properties
Lower acids with up to 3 carbon atoms are volatile, colorless liquids with a characteristic pungent odor, miscible with water in any ratio. Most acids C 4 - C 9 are oily liquids with an unpleasant odor. Solubility in water greatly decreases with increasing molar mass. Acids from C 10 and above are solids that are insoluble in water. The densities of formic and acetic acids are greater than one, the rest are less than one. The boiling point increases with increasing molar mass, with the same number of carbon atoms, acids of a normal structure boil higher than acids with a branched carbon skeleton. A comparison of the boiling points of acids and alcohols with the same number of carbon atoms showed that acids boil at much higher temperatures than alcohols. This indicates a higher association of acid molecules compared to alcohols due to the formation of hydrogen bonds.
Carboxylic acids, like alcohols, are capable of forming hydrogen bonds. If the acceptor is a sufficiently strong base, the formation of a hydrogen bond precedes the complete transfer of a proton to the base. According to Bronsted, a compound that is a hydrogen donor is considered an "acid". Whether a given compound will be a “hydrogen donor” (“acid”) depends on the nature of the “hydrogen acceptor” (“base”). The stronger the base, the more likely it is that the compound will behave like an acid towards it:

The intermolecular hydrogen bonds that arise between the molecules of carboxylic acids are so strong that even in the gaseous state, a significant part of the molecules exist in the form of dimers:

As the hydrocarbon chain grows, the ability of acids to form hydrogen bonds decreases.
DEFINITION
Organic substances whose molecules contain one or more carboxyl groups connected to a hydrocarbon radical are called carboxylic acids.
The first three members of the homologous series of carboxylic acids, including propionic acid, are liquids that have a pungent odor and are highly soluble in water. The following homologues, starting with butyric acid, are also liquids that have a sharp unpleasant odor, but are poorly soluble in water. Higher acids, with a carbon number of 10 or more, are solids, odorless, insoluble in water. In general, in the series of homologues, with increasing molecular weight, the solubility in water decreases, the density decreases, and the boiling point increases (Table 1).
Table 1. Homologous series of carboxylic acids.
Obtaining carboxylic acids
Carboxylic acids are obtained by oxidation of saturated hydrocarbons, alcohols, aldehydes. For example, acetic acid - by oxidizing ethanol with a solution of potassium permanganate in an acidic medium when heated:
Chemical properties of carboxylic acids
The chemical properties of carboxylic acids are primarily due to the peculiarities of their structure. So, water-soluble acids are able to dissociate into ions:
R-COOH↔R-COO - + H + .
Due to the presence of the H + ion in water, they have a sour taste, are able to change the color of the indicators and conduct electricity. In aqueous solution, these acids are weak electrolytes.
Carboxylic acids have chemical properties characteristic of solutions of inorganic acids, i.e. interact with metals (1), their oxides (2), hydroxides (3) and weak salts (4):
2CH 3 -COOh + Zn → (CH 3 COO) 2 Zn + H 2 (1);
2CH 3 -COOH + CuO→ (CH 3 COO) 2 Cu + H 2 O (2);
R-COOH + KOH → R-COOK + H 2 O (3);
2CH 3 -COOH + NaHCO 3 → CH 3 COONa + H 2 O + CO 2 (4).
A specific property of limiting, as well as unsaturated carboxylic acids, manifested due to the functional group, is interaction with alcohols.
Carboxylic acids react with alcohols when heated and in the presence of concentrated sulfuric acid. For example, if ethyl alcohol and a little sulfuric acid are added to acetic acid, then when heated, the smell of ethyl ester of acetic acid (ethyl acetate) appears:
CH 3 -COOH + C 2 H 5 OH ↔CH 3 -C (O) -O-C 2 H 5 + H 2 O.
A specific property of saturated carboxylic acids, manifested due to the radical, is the halogenation (chlorination) reaction.

Application of carboxylic acids
Carboxylic acids serve as feedstock for the production of ketones, acid halides, vinyl esters, and other important classes of organic compounds.
Formic acid is widely used to obtain esters used in perfumery, leather (tanning), textile industry (as a mordant in dyeing), as a solvent and preservative.
An aqueous solution (70-80%) of acetic acid is called vinegar essence, and a 3-9% aqueous solution is called table vinegar. The essence is often used to make vinegar at home by dilution.
Examples of problem solving
EXAMPLE 1
| Exercise | What chemical reactions can be used to carry out the following transformations: a) CH 4 → CH 3 Cl → CH 3 OH → HCHO → HCOOH → HCOOK. Write the reaction equations, indicate the conditions for their occurrence. |
| Answer | a) Chlorination of methane in the presence of light leads to the production of chloromethane: CH 4 + Cl 2 →CH 3 Cl + HCl. Halogen derivatives of alkanes undergo hydrolysis in an aqueous or alkaline medium with the formation of alcohols: CH 3 Cl + NaOH→CH 3 OH + NaCl. As a result of the oxidation of primary alcohols, for example, with potassium dichromate in an acidic medium in the presence of a catalyst (Cu, CuO, Pt, Ag), aldehydes are formed: CH 3 OH+ [O] →HCHO. Aldehydes are easily oxidized to the corresponding carboxylic acids, for example, with potassium permanganate: HCHO + [O]→HCOOH. Carboxylic acids exhibit all the properties inherent in weak mineral acids, i.e. able to interact with active metals to form salts: 2HCOOH+ 2K→2HCOOK + H2. |
EXAMPLE 2
| Exercise | Write the reaction equations between the following substances: a) 2-methylpropanoic acid and chlorine; b) acetic acid and propanol-2; c) acrylic acid and bromine water; d) 2-methylbutanoic acid and phosphorus (V) chloride. Specify the reaction conditions. |
| Answer | a) as a result of the interaction reaction between 2-methylpropanoic acid and chlorine, the hydrogen atom is replaced in the hydrocarbon radical located in the a-position; 2-methyl-2-chloropropanoic acid is formed H 3 C-C (CH 3) H-COOH + Cl 2 → H 3 C-C (CH 3) Cl-COOH + HCl (kat \u003d P). b) as a result of the reaction of interaction between acetic acid and propanol-2, an ester is formed - isopropyl ester of acetic acid. CH 3 -COOH + CH 3 -C (OH) H-CH 3 → CH 3 -C (O) -O-C (CH 3) -CH 3. c) as a result of the interaction reaction between acrylic acid and bromine water, the addition of a halogen at the double bond site in accordance with Markovnikov's rule; 2,3-dibromopropanoic acid is formed CH 2 \u003d CH-COOH + Br 2 → CH 2 Br-CHBr-COOH d) as a result of the interaction reaction between 2-methylbutanoic acid and phosphorus (V) chloride, the corresponding acid chloride is formed CH 3 -CH 2 -C (CH 3) H-COOH + PCl 5 →CH 3 -CH 2 -C (CH 3) H-COOCl + POCl 3 + HCl. |
Classification
a) By basicity (i.e., the number of carboxyl groups in a molecule):
Monobasic (monocarboxylic) RCOOH; For example:
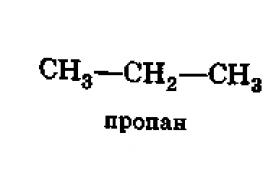
CH 3 CH 2 CH 2 COOH;
HOOS-CH 2 -COOH propanedioic (malonic) acid

Tribasic (tricarboxylic) R (COOH) 3, etc.
b) According to the structure of the hydrocarbon radical:
Aliphatic
limit; for example: CH 3 CH 2 COOH;
unsaturated; for example: CH 2 \u003d CHCOOH propenoic (acrylic) acid

Alicyclic, for example:

Aromatic, for example:

Limit monocarboxylic acids
(monobasic saturated carboxylic acids) - carboxylic acids in which a saturated hydrocarbon radical is connected to one carboxyl group -COOH. They all have the general formula C n H 2n+1 COOH (n ≥ 0); or CnH 2n O 2 (n≥1)
Nomenclature
The systematic names of monobasic saturated carboxylic acids are given by the name of the corresponding alkane with the addition of the suffix -ovaya and the word acid.
1. HCOOH methane (formic) acid
2. CH 3 COOH ethanoic (acetic) acid
3. CH 3 CH 2 COOH propanoic (propionic) acid
isomerism
The isomerism of the skeleton in the hydrocarbon radical is manifested, starting with butanoic acid, which has two isomers:

Interclass isomerism manifests itself, starting with acetic acid:
CH 3 -COOH acetic acid;
H-COO-CH 3 methyl formate (methyl ester of formic acid);
HO-CH 2 -COH hydroxyethanal (hydroxyacetic aldehyde);
HO-CHO-CH 2 hydroxyethylene oxide.
homologous series
Trivial name |
IUPAC name |
|
Formic acid |
Methanoic acid |
|
Acetic acid |
Ethanoic acid |
|
propionic acid |
propanoic acid |
|
Butyric acid |
Butanoic acid |
|
Valeric acid |
Pentanoic acid |
|
Caproic acid |
Hexanoic acid |
|
Enanthic acid |
Heptanoic acid |
|
Caprylic acid |
Octanoic acid |
|
Pelargonic acid |
Nonanoic acid |
|
capric acid |
Decanoic acid |
|
Undecylic acid |
undecanoic acid |
|
Palmitic acid |
Hexadecanic acid |
|
Stearic acid |
Octadecanic acid |
Acid residues and acid radicals
acid residue |
Acid radical (acyl) |
|
UNSD |
NSOO- |
|
CH 3 COOH |
CH 3 SOO- |
|
CH 3 CH 2 COOH |
CH 3 CH 2 COO- |
|
CH 3 (CH 2) 2 COOH |
CH 3 (CH 2) 2 COO- |
|
CH 3 (CH 2) 3 COOH |
CH 3 (CH 2) 3 COO- |
|
CH 3 (CH 2) 4 COOH |
CH 3 (CH 2) 4 COO- |
Electronic structure of carboxylic acid molecules

The shift of electron density shown in the formula towards the carbonyl oxygen atom causes a strong polarization of the O-H bond, as a result of which the detachment of the hydrogen atom in the form of a proton is facilitated - in aqueous solutions, the process of acid dissociation occurs:
RCOOH ↔ RCOO - + H +
In the carboxylate ion (RCOO -), p, π-conjugation of the lone pair of electrons of the oxygen atom of the hydroxyl group with p-clouds forming a π-bond takes place, as a result, the π-bond is delocalized and the negative charge is uniformly distributed between the two oxygen atoms:

In this regard, for carboxylic acids, in contrast to aldehydes, addition reactions are not characteristic.
Physical Properties

The boiling points of acids are much higher than the boiling points of alcohols and aldehydes with the same number of carbon atoms, which is explained by the formation of cyclic and linear associates between acid molecules due to hydrogen bonds:
Chemical properties
I. Acid properties
The strength of acids decreases in the series:
HCOOH → CH 3 COOH → C 2 H 6 COOH → ...
1. Neutralization reactions
CH 3 COOH + KOH → CH 3 COOK + n 2 O
2. Reactions with basic oxides
2HCOOH + CaO → (HCOO) 2 Ca + H 2 O
3. Reactions with metals
2CH 3 CH 2 COOH + 2Na → 2CH 3 CH 2 COONa + H 2
4. Reactions with salts of weaker acids (including carbonates and bicarbonates)
2CH 3 COOH + Na 2 CO 3 → 2CH 3 COONa + CO 2 + H 2 O
2HCOOH + Mg(HCO 3) 2 → (HCOO) 2 Mg + 2CO 2 + 2H 2 O
(HCOOH + HCO 3 - → HCOO - + CO2 + H2O)
5. Reactions with ammonia
CH 3 COOH + NH 3 → CH 3 COONH 4
II. -OH group substitution
1. Interaction with alcohols (esterification reactions)

2. Interaction with NH 3 when heated (acid amides are formed)

Acid amides  hydrolyzed to form acids:
hydrolyzed to form acids:

or their salts:

3. Formation of acid halides
Acid chlorides are of the greatest importance. Chlorinating reagents - PCl 3 , PCl 5 , thionyl chloride SOCl 2 .

4. Formation of acid anhydrides (intermolecular dehydration)

Acid anhydrides are also formed by the interaction of acid chlorides with anhydrous salts of carboxylic acids; in this case, mixed anhydrides of various acids can be obtained; For example:

III. Substitution reactions of hydrogen atoms at the α-carbon atom

Features of the structure and properties of formic acid
The structure of the molecule

The formic acid molecule, unlike other carboxylic acids, contains an aldehyde group in its structure.
Chemical properties
Formic acid enters into reactions characteristic of both acids and aldehydes. Showing the properties of an aldehyde, it is easily oxidized to carbonic acid:
In particular, HCOOH is oxidized with an ammonia solution of Ag 2 O and copper (II) hydroxide Сu (OH) 2, i.e. gives qualitative reactions to the aldehyde group:

When heated with concentrated H 2 SO 4, formic acid decomposes into carbon monoxide (II) and water:

Formic acid is noticeably stronger than other aliphatic acids, since the carboxyl group in it is bonded to a hydrogen atom, and not to an electron-donating alkyl radical.
Methods for obtaining saturated monocarboxylic acids
1. Oxidation of alcohols and aldehydes
The general scheme for the oxidation of alcohols and aldehydes:
KMnO 4 , K 2 Cr 2 O 7 , HNO 3 and other reagents are used as oxidizers.
For example:
5C 2 H 5 OH + 4KMnO 4 + 6H 2 S0 4 → 5CH 3 COOH + 2K 2 SO 4 + 4MnSO 4 + 11H 2 O
2. Hydrolysis of esters

3. Oxidative cleavage of double and triple bonds in alkenes and alkynes

Methods for obtaining HCOOH (specific)
1. Interaction of carbon monoxide (II) with sodium hydroxide
CO + NaOH → HCOONa sodium formate
2HCOONa + H 2 SO 4 → 2HCOOH + Na 2 SO 4
2. Decarboxylation of oxalic acid

Methods for obtaining CH 3 COOH (specific)
1. Catalytic oxidation of butane

2. Synthesis from acetylene

3. Catalytic carbonylation of methanol

4. Acetic acid fermentation of ethanol
This is how food grade acetic acid is obtained.
Obtaining higher carboxylic acids
Hydrolysis of natural fats

Unsaturated monocarboxylic acids
Key Representatives
General formula of alkenoic acids: C n H 2n-1 COOH (n ≥ 2)
CH 2 \u003d CH-COOH propenoic (acrylic) acid

Higher unsaturated acids
The radicals of these acids are part of vegetable oils.
C 17 H 33 COOH - oleic acid, or cis-octadiene-9-oic acid

Trance-isomer of oleic acid is called elaidic acid.
C 17 H 31 COOH - linoleic acid, or cis, cis-octadiene-9,12-oic acid

C 17 H 29 COOH - linolenic acid, or cis, cis, cis-octadecatriene-9,12,15-oic acid
In addition to the general properties of carboxylic acids, unsaturated acids are characterized by addition reactions at multiple bonds in the hydrocarbon radical. So, unsaturated acids, like alkenes, are hydrogenated and decolorize bromine water, for example:

Individual representatives of dicarboxylic acids
Limiting dicarboxylic acids HOOC-R-COOH
HOOC-CH 2 -COOH propanedioic (malonic) acid, (salts and esters - malonates)
HOOC-(CH 2) 2 -COOH butadiic (succinic) acid, (salts and esters - succinates)
HOOC-(CH 2) 3 -COOH pentadiic (glutaric) acid, (salts and esters - glutorates)
HOOC-(CH 2) 4 -COOH hexadioic (adipic) acid, (salts and esters - adipinates)
Features of chemical properties
Dicarboxylic acids are in many ways similar to monocarboxylic acids, but are stronger. For example, oxalic acid is almost 200 times stronger than acetic acid.
Dicarboxylic acids behave like dibasic acids and form two series of salts - acidic and medium:
HOOC-COOH + NaOH → HOOC-COONa + H 2 O
HOOC-COOH + 2NaOH → NaOOC-COONa + 2H 2 O
When heated, oxalic and malonic acids are easily decarboxylated: