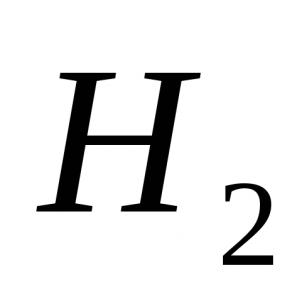Iron salts 3 color. Chemical properties of iron and its compounds, their application
Formula:
Iron(II) sulfate, ferrous sulfate, FeSO 4 - a salt of sulfuric acid and 2-valent iron. Hardness - 2.
In chemistry, iron sulphate is called crystalline hydrate iron(II) sulfate. Light green crystals. It is used in the textile industry, in agriculture as an insecticide, for the preparation of mineral paints.
Natural analogue - mineral melanterite; in nature, it occurs in crystals of the monoclinohedral system, green-yellow in color, in the form of smears or streaks.
Molar mass: 151.91 g/mol
Density: 1.8-1.9 g/cm³
Melting temperature: 400°C
Solubility in water: 25.6 g/100 ml
Sulphate of 2-valent iron is released at temperatures from 1.82 ° C to 56.8 ° C from aqueous solutions in the form of light green crystals of FeSO 4 7H 2 O, is called ferrous sulfate (crystal hydrate) in technology. Dissolves in 100 g of water: 26.6 g of anhydrous FeSO 4 at 20 ° C and 54.4 g at 56 ° C.
Solutions of sulfate of 2-valent iron under the influence of atmospheric oxygen oxidize over time, turning into iron (III) sulfate:
12FeSO 4 + O 2 + 6H 2 O \u003d 4Fe 2 (SO 4) 3 + 4Fe (OH) 3 ↓
When heated above 480 °C, it decomposes:
2FeSO 4 → Fe 2 O 3 + SO 2 + SO 3
Receipt.
Ferrous vitriol can be prepared by the action of dilute sulfuric acid on scrap iron, roofing iron cuttings, etc. In industry, it is obtained as a by-product when iron sheets, wire, etc. are etched with dilute H 2 SO 4 to remove scale.
Fe + H 2 SO 4 \u003d FeSO 4 + H 2
Another way is oxidative roasting of pyrite:
2FeS 2 + 7O 2 + 2H 2 O \u003d 2FeSO 4 + 2H 2 SO 4
Qualitative analysis.
Analytical reactions for the iron cation (II).
1. With potassium hexacyanoferrate(III) K 3 with the formation of a dark blue precipitate of potassium iron(II) hexacyanoferrate(III) (“turnbull blue”), insoluble in acids, decomposing with alkalis to form Fe(OH) 3 (HF).
FeSO 4 + K 3 KFe + K 2 SO 4
The optimum pH value for the reaction is 2-3. The reaction is fractional, highly sensitive. High concentrations of Fe 3+ interfere.
2. With ammonium sulfide (NH 4 ) 2 S with the formation of a black precipitate, soluble in strong acids (HF).
FeSO 4
+ (NH 4) 2 S  FeS + (NH 4) 2 SO 4
FeS + (NH 4) 2 SO 4
3.2. Analytical reactions for sulfate ion.
1. With the group reagent BaCl 2 + CaCl 2 or BaCl 2 (HF).
Fractional opening of the sulfate ion is carried out in an acidic environment, which eliminates the interfering effect of CO 3 2-, PO 4 3-, etc., and by boiling the test solution with 6 mol / dm 3 HCl to remove S 2-, SO 3 2 -, S 2 O 3 2- -ions, which can form elemental sulfur, the precipitate of which can be taken as a precipitate of BaSO 4 . The BaSO 4 precipitate is able to form isomorphic crystals with KMnO 4 and turn pink (the specificity of the reaction increases).
Methodology performing the reaction in the presence of 0.002 mol/dm 3 KMnO 4 .
To 3-5 drops of the test solution, add equal volumes of solutions of potassium permanganate, barium chloride and hydrochloric acid and mix vigorously for 2-3 minutes. Allow to settle and, without separating the precipitate from the solution, add 1-2 drops of a 3% solution of H 2 O 2 , mix and centrifuge. The precipitate should remain pink in color and the solution above the precipitate should become colorless.
2. With lead acetate.
SO 4
2-
+Pb2+  PbSO 4
PbSO 4
Methodology : to 2 cm 3 of a sulfate solution add 0.5 cm 3 of dilute hydrochloric acid and 0.5 cm 3 of a lead acetate solution; a white precipitate is formed, soluble in a saturated solution of ammonium acetate or sodium hydroxide.
PbSO 4 + 4 NaOH  Na 2 + Na 2 SO 4
Na 2 + Na 2 SO 4
With strontium salts - the formation of a white precipitate, insoluble in acids (unlike sulfites).
SO 4
2
-+Sr2+  SrSO 4
SrSO 4
Methodology : To 4-5 drops of the analyzed solution add 4-5 drops of a concentrated solution of strontium chloride, a white precipitate is formed.
With calcium salts - the formation of needle-like crystals of gypsum CaSO 4 2H 2 O.
SO 4 2- + Ca 2+ + 2H 2 O
 CaSO 4 2H 2 O
CaSO 4 2H 2 O
Methodology: put a drop of the analyzed solution and calcium salts on a glass slide, slightly dry. The formed crystals are examined under a microscope.
Quantitative analysis.
Permanganatometry.
Determination of the mass fraction of iron in a sample of Mohr's salt (NH 4) 2 Fe (SO 4) 2 6H 2 O by the permanganometric method
(direct titration option)
The definition is based on the oxidation of iron(II) by potassium permanganate to iron(III).
10 FeSO 4 + 2 KMnO 4 + 8H 2 SO 4 = 5 Fe 2 (SO 4 ) 3 + 2 MnSO 4 + K 2 SO 4 + 8H 2 O
M (Fe) = 55.85 g/mol
Methodology: The exact weight of Mohr's salt, necessary for the preparation of 100 cm 3 of a 0.1 M solution of Mohr's salt, is quantitatively transferred into a volumetric flask with a capacity of 100 cm 3, dissolved in a small amount of distilled water, after complete dissolution, brought to the mark with water, mixed. An aliquot of the resulting solution (individual task) is placed in a titration flask, an equal volume of diluted sulfuric acid (1:5) is added and slowly titrated with a solution of potassium permanganate until a slightly pink color of the solution is stable for 30 seconds.
Application.
Used in production ink;
In the dye business (for coloring wool in black color)
For tree conservation.
Bibliography.
- Ordinal number - 26.
- The period is the fourth big one.
- The eighth group, the secondary subgroup.
- The atomic weight is 55.847.
- The structure of the outer electron shell is denoted by the formula 3d 6 4s 2 .
- - Fe.
- The name is iron, the reading in the formula is "ferrum".
- In nature, there are four stable isotopes of the element in question with mass numbers 54, 56, 57, 58.
- melting point - 1539 0 С;
- boiling - 2862 0 C;
- activity - average;
- refractoriness - high;
- exhibits pronounced magnetic properties.
- acids;
- oxygen (including air);
- gray;
- halogens;
- when heated - with nitrogen, phosphorus, carbon and silicon;
- with salts of less active metals, reducing them to simple substances;
- with sharp water vapor;
- with iron salts in the oxidation state +3.
- The cores of the terrestrial planets - 90%.
- In the earth's crust - 5%.
- In the Earth's mantle - 12%.
- In the earth's core - 86%.
- In river water - 2 mg/l.
- In the sea and ocean - 0.02 mg / l.
- magnetite;
- limonite or brown iron ore;
- vivianite;
- pyrrhotite;
- pyrite;
- siderite;
- marcasite;
- lellingite;
- mispikel;
- milanterite and others.
- cast irons;
- become.
- oxide;
- hydroxide;
- binary compounds;
- complex salts;
- complex compounds.
- Iron(II) oxide. Black powder, insoluble in water. The nature of the connection is basic. It is able to quickly oxidize, however, it can also be easily reduced to a simple substance. It dissolves in acids to form the corresponding salts. Formula - FeO.
- Iron(II) hydroxide. It is a white amorphous precipitate. Formed by the reaction of salts with bases (alkalis). It shows weak basic properties, is able to quickly oxidize in air to iron compounds +3. Formula - Fe (OH) 2.
- The salts of an element in the specified oxidation state. As a rule, they have a pale green color of the solution, oxidize well even in air, acquiring and turning into iron salts 3. They dissolve in water. Examples of compounds: FeCL 2 , FeSO 4 , Fe(NO 3) 2 .

Several compounds are of practical importance among the designated substances. First, (II). This is the main supplier of ions to the human body with anemia. When such an ailment is diagnosed in a patient, he is prescribed complex preparations, which are based on the compound in question. This is how iron deficiency in the body is replenished.
Secondly, that is, iron (II) sulfate, together with copper, is used to destroy agricultural pests in crops. The method has been proving its effectiveness for more than a dozen years, therefore it is very much appreciated by gardeners and gardeners.
Mora Salt
This is a compound that is a crystalline hydrate of iron and ammonium sulfate. Its formula is written as FeSO 4 * (NH 4) 2 SO 4 * 6H 2 O. One of the iron (II) compounds, which has been widely used in practice. The main areas of human use are as follows.
- Pharmaceutics.
- Scientific research and laboratory titrimetric analyzes (to determine the content of chromium, potassium permanganate, vanadium).
- Medicine - as an additive to food with a lack of iron in the patient's body.
- For impregnation of wooden products, as Mora salt protects against decay processes.
There are other areas in which this substance finds application. It got its name in honor of the German chemist who first discovered the manifested properties.
Substances with an oxidation state of iron (III)
The properties of iron compounds, in which it exhibits an oxidation state of +3, are somewhat different from those discussed above. Thus, the nature of the corresponding oxide and hydroxide is no longer basic, but pronounced amphoteric. We give a description of the main substances.

Among the examples given, from a practical point of view, such a crystalline hydrate as FeCL 3 * 6H 2 O, or iron (III) chloride hexahydrate, is important. It is used in medicine to stop bleeding and replenish iron ions in the body with anemia.
Iron(III) sulfate pentahydrate is used to purify drinking water, as it behaves as a coagulant.
Iron(VI) compounds
The formulas of the chemical compounds of iron, where it exhibits a special oxidation state of +6, can be written as follows:
- K 2 FeO 4 ;
- Na 2 FeO 4 ;
- MgFeO 4 and others.
All of them have a common name - ferrates - and have similar properties (strong reducing agents). They are also able to disinfect and have a bactericidal effect. This allows them to be used for the treatment of drinking water on an industrial scale.
Complex compounds
Special substances are very important in analytical chemistry and not only. Those that form in aqueous solutions of salts. These are complex compounds of iron. The most popular and well-studied of them are as follows.
- Potassium hexacyanoferrate (II) K 4 . Another name for the compound is yellow blood salt. It is used for qualitative determination of iron ion Fe 3+ in solution. As a result of exposure, the solution acquires a beautiful bright blue color, since another complex is formed - Prussian blue KFe 3+. Since ancient times it has been used as
- Potassium hexacyanoferrate (III) K 3 . Another name is red blood salt. It is used as a qualitative reagent for the determination of iron ions Fe 2+ . As a result, a blue precipitate is formed, which is called Turnbull blue. Also used as a dye for fabric.

Iron in organic matter
Iron and its compounds, as we have already seen, are of great practical importance in the economic life of man. However, in addition to this, its biological role in the body is no less great, on the contrary.
There is one very important protein, which includes this element. This is hemoglobin. It is thanks to him that oxygen is transported and uniform and timely gas exchange is carried out. Therefore, the role of iron in the vital process - respiration - is simply enormous.

In total, the human body contains about 4 grams of iron, which must be constantly replenished through the food consumed.
- Chemical formulas must be entered case sensitive
- Indexes are entered as regular numbers
- The dot on the midline (multiplication sign), used, for example, in the formulas of crystalline hydrates, is replaced by a regular dot.
- Example: instead of CuSO₄ 5H₂O, the converter uses the spelling CuSO4.5H2O for ease of entry.
- salt (sodium chloride) NaCl
- sugar (sucrose) C₁₂H₂₂O₁₁
- vinegar (acetic acid solution) CH₃COOH
- determine the atomic masses of the elements according to the periodic table;
- determine the number of atoms of each element in the compound formula;
- determine the molar mass by adding the atomic masses of the elements included in the compound, multiplied by their number.
- two carbon atoms
- four hydrogen atoms
- two oxygen atoms
- carbon C = 2 × 12.0107 g/mol = 24.0214 g/mol
- hydrogen H = 4 × 1.00794 g/mol = 4.03176 g/mol
- oxygen O = 2 × 15.9994 g/mol = 31.9988 g/mol
- molar mass = 24.0214 + 4.03176 + 31.9988 = 60.05196 g/mol
Lurie Yu.Yu. Handbook of analytical chemistry. Moscow, 1972;
Guideline "Instrumental Methods of Analysis", Perm, 2004;
Guideline "Qualitative chemical analysis", Perm, 2003;
Guideline "Quantitative chemical analysis", Perm, 2004;
Rabinovich V.A., Khavin Z.Ya. Brief chemical reference book, Leningrad, 1991;
"Great Soviet Encyclopedia";
DEFINITION
Iron- an element of the eighth group of the fourth period of the Periodic system of chemical elements of D. I. Mendeleev.
And the languid number is 26. The symbol is Fe (lat. “ferrum”). One of the most common metals in the earth's crust (second place after aluminum).
Physical properties of iron
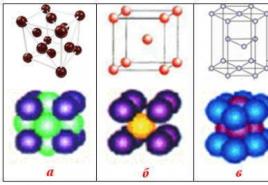
Iron is a gray metal. In its pure form, it is quite soft, malleable and ductile. The electronic configuration of the external energy level is 3d 6 4s 2 . In its compounds, iron exhibits the oxidation states "+2" and "+3". The melting point of iron is 1539C. Iron forms two crystalline modifications: α- and γ-iron. The first of them has a cubic body-centered lattice, the second has a cubic face-centered one. α-Iron is thermodynamically stable in two temperature ranges: below 912 and from 1394C to the melting point. Between 912 and 1394C, γ-iron is stable.
The mechanical properties of iron depend on its purity - the content in it of even very small amounts of other elements. Solid iron has the ability to dissolve many elements in itself.
Chemical properties of iron
In moist air, iron quickly rusts, i.e. covered with a brown coating of hydrated iron oxide, which, due to its friability, does not protect iron from further oxidation. In water, iron corrodes intensively; with abundant access of oxygen, hydrated forms of iron oxide (III) are formed:
2Fe + 3/2O 2 + nH 2 O = Fe 2 O 3 × H 2 O.
With a lack of oxygen or with difficult access, a mixed oxide (II, III) Fe 3 O 4 is formed:
3Fe + 4H 2 O (v) ↔ Fe 3 O 4 + 4H 2.
Iron dissolves in hydrochloric acid of any concentration:
Fe + 2HCl \u003d FeCl 2 + H 2.
Similarly, dissolution occurs in dilute sulfuric acid:
Fe + H 2 SO 4 \u003d FeSO 4 + H 2.
In concentrated solutions of sulfuric acid, iron is oxidized to iron (III):
2Fe + 6H 2 SO 4 \u003d Fe 2 (SO 4) 3 + 3SO 2 + 6H 2 O.
However, in sulfuric acid, the concentration of which is close to 100%, iron becomes passive and there is practically no interaction. In dilute and moderately concentrated solutions of nitric acid, iron dissolves:
Fe + 4HNO 3 \u003d Fe (NO 3) 3 + NO + 2H 2 O.
At high concentrations of nitric acid, dissolution slows down and iron becomes passive.
Like other metals, iron reacts with simple substances. The reactions of the interaction of iron with halogens (regardless of the type of halogen) proceed when heated. The interaction of iron with bromine proceeds at an increased vapor pressure of the latter:
2Fe + 3Cl 2 \u003d 2FeCl 3;
3Fe + 4I 2 = Fe 3 I 8.
The interaction of iron with sulfur (powder), nitrogen and phosphorus also occurs when heated:
6Fe + N 2 = 2Fe 3 N;
2Fe + P = Fe 2 P;
3Fe + P = Fe 3 P.
Iron is able to react with non-metals such as carbon and silicon:
3Fe + C = Fe 3 C;
Among the reactions of interaction of iron with complex substances, the following reactions play a special role - iron is able to reduce metals that are in the activity series to the right of it, from salt solutions (1), to reduce iron (III) compounds (2):
Fe + CuSO 4 \u003d FeSO 4 + Cu (1);
Fe + 2FeCl 3 = 3FeCl 2 (2).
Iron, at elevated pressure, reacts with a non-salt-forming oxide - CO to form substances of complex composition - carbonyls - Fe (CO) 5, Fe 2 (CO) 9 and Fe 3 (CO) 12.
Iron, in the absence of impurities, is stable in water and in dilute alkali solutions.
Getting iron
The main way to obtain iron is from iron ore (hematite, magnetite) or electrolysis of solutions of its salts (in this case, “pure” iron is obtained, i.e. iron without impurities).
Examples of problem solving
EXAMPLE 1
| Exercise | Iron scale Fe 3 O 4 weighing 10 g was first treated with 150 ml of hydrochloric acid solution (density 1.1 g/ml) with a mass fraction of hydrogen chloride of 20%, and then an excess of iron was added to the resulting solution. Determine the composition of the solution (in % by weight). |
| Decision | We write the reaction equations according to the condition of the problem: 8HCl + Fe 3 O 4 \u003d FeCl 2 + 2FeCl 3 + 4H 2 O (1); 2FeCl 3 + Fe = 3FeCl 2 (2). Knowing the density and volume of a hydrochloric acid solution, you can find its mass: m sol (HCl) = V(HCl) × ρ (HCl); m sol (HCl) \u003d 150 × 1.1 \u003d 165 g. Calculate the mass of hydrogen chloride: m(HCl)=msol(HCl)×ω(HCl)/100%; m(HCl) = 165 x 20%/100% = 33 g. The molar mass (mass of one mol) of hydrochloric acid, calculated using the table of chemical elements of D.I. Mendeleev - 36.5 g / mol. Find the amount of hydrogen chloride substance: v(HCl) = m(HCl)/M(HCl); v (HCl) \u003d 33 / 36.5 \u003d 0.904 mol. Molar mass (mass of one mole) of scale, calculated using the table of chemical elements of D.I. Mendeleev - 232 g/mol. Find the amount of scale substance: v (Fe 3 O 4) \u003d 10/232 \u003d 0.043 mol. According to equation 1, v(HCl): v(Fe 3 O 4) \u003d 1: 8, therefore, v (HCl) \u003d 8 v (Fe 3 O 4) \u003d 0.344 mol. Then, the amount of hydrogen chloride substance calculated according to the equation (0.344 mol) will be less than that indicated in the condition of the problem (0.904 mol). Therefore, hydrochloric acid is in excess and another reaction will proceed: Fe + 2HCl = FeCl 2 + H 2 (3). Let's determine the amount of iron chloride substance formed as a result of the first reaction (indices denote a specific reaction): v 1 (FeCl 2): v (Fe 2 O 3) = 1:1 = 0.043 mol; v 1 (FeCl 3): v (Fe 2 O 3) = 2:1; v 1 (FeCl 3) = 2 × v (Fe 2 O 3) = 0.086 mol. Let's determine the amount of hydrogen chloride that did not react in reaction 1 and the amount of iron (II) chloride substance formed during reaction 3: v rem (HCl) \u003d v (HCl) - v 1 (HCl) \u003d 0.904 - 0.344 \u003d 0.56 mol; v 3 (FeCl 2): v rem (HCl) = 1:2; v 3 (FeCl 2) \u003d 1/2 × v rem (HCl) \u003d 0.28 mol. Let's determine the amount of FeCl 2 substance formed during reaction 2, the total amount of FeCl 2 substance and its mass: v 2 (FeCl 3) = v 1 (FeCl 3) = 0.086 mol; v 2 (FeCl 2): v 2 (FeCl 3) = 3:2; v 2 (FeCl 2) = 3/2× v 2 (FeCl 3) = 0.129 mol; v sum (FeCl 2) \u003d v 1 (FeCl 2) + v 2 (FeCl 2) + v 3 (FeCl 2) \u003d 0.043 + 0.129 + 0.28 \u003d 0.452 mol; m (FeCl 2) \u003d v sum (FeCl 2) × M (FeCl 2) \u003d 0.452 × 127 \u003d 57.404 g. Let us determine the amount of substance and the mass of iron that entered into reactions 2 and 3: v 2 (Fe): v 2 (FeCl 3) = 1:2; v 2 (Fe) \u003d 1/2 × v 2 (FeCl 3) \u003d 0.043 mol; v 3 (Fe): v rem (HCl) = 1:2; v 3 (Fe) = 1/2×v rem (HCl) = 0.28 mol; v sum (Fe) \u003d v 2 (Fe) + v 3 (Fe) \u003d 0.043 + 0.28 \u003d 0.323 mol; m(Fe) = v sum (Fe) ×M(Fe) = 0.323 ×56 = 18.088 g. Let us calculate the amount of substance and the mass of hydrogen released in reaction 3: v (H 2) \u003d 1/2 × v rem (HCl) \u003d 0.28 mol; m (H 2) \u003d v (H 2) × M (H 2) \u003d 0.28 × 2 \u003d 0.56 g. We determine the mass of the resulting solution m ' sol and the mass fraction of FeCl 2 in it: m’ sol \u003d m sol (HCl) + m (Fe 3 O 4) + m (Fe) - m (H 2); |
The human body contains about 5 g of iron, most of it (70%) is part of the hemoglobin in the blood.
Physical Properties
In the free state, iron is a silvery-white metal with a grayish tint. Pure iron is ductile and has ferromagnetic properties. In practice, iron alloys are commonly used - cast irons and steels.
Fe is the most important and most common element of the nine d-metals of the secondary subgroup of group VIII. Together with cobalt and nickel, it forms the "iron family".
When forming compounds with other elements, it often uses 2 or 3 electrons (B \u003d II, III).
Iron, like almost all d-elements of group VIII, does not show a higher valency equal to the group number. Its maximum valency reaches VI and is extremely rare.
The most typical compounds are those in which the Fe atoms are in the +2 and +3 oxidation states.
Methods for obtaining iron
1. Commercial iron (in an alloy with carbon and other impurities) is obtained by carbothermal reduction of its natural compounds according to the scheme:

Recovery occurs gradually, in 3 stages:
1) 3Fe 2 O 3 + CO = 2Fe 3 O 4 + CO 2
2) Fe 3 O 4 + CO = 3FeO + CO 2
3) FeO + CO \u003d Fe + CO 2
The cast iron resulting from this process contains more than 2% carbon. In the future, steels are obtained from cast iron - iron alloys containing less than 1.5% carbon.
2. Very pure iron is obtained in one of the following ways:
a) decomposition of pentacarbonyl Fe
Fe(CO) 5 = Fe + 5CO
b) hydrogen reduction of pure FeO
FeO + H 2 \u003d Fe + H 2 O
c) electrolysis of aqueous solutions of Fe +2 salts
FeC 2 O 4 \u003d Fe + 2СO 2
iron(II) oxalate
Chemical properties
Fe - a metal of medium activity, exhibits general properties characteristic of metals.
A unique feature is the ability to "rust" in humid air:
In the absence of moisture with dry air, iron begins to noticeably react only at T > 150°C; when calcined, “iron scale” Fe 3 O 4 is formed:
3Fe + 2O 2 = Fe 3 O 4
Iron does not dissolve in water in the absence of oxygen. At very high temperatures, Fe reacts with water vapor, displacing hydrogen from water molecules:
3 Fe + 4H 2 O (g) \u003d 4H 2
The rusting process in its mechanism is electrochemical corrosion. The rust product is presented in a simplified form. In fact, a loose layer of a mixture of oxides and hydroxides of variable composition is formed. Unlike the Al 2 O 3 film, this layer does not protect the iron from further destruction.
Types of corrosion

Corrosion protection of iron

1. Interaction with halogens and sulfur at high temperature.
2Fe + 3Cl 2 = 2FeCl 3
2Fe + 3F 2 = 2FeF 3
Fe + I 2 \u003d FeI 2
Compounds are formed in which the ionic type of bond predominates.
2. Interaction with phosphorus, carbon, silicon (iron does not directly combine with N 2 and H 2, but dissolves them).
Fe + P = Fe x P y
Fe + C = Fe x C y
Fe + Si = FexSiy
Substances of variable composition are formed, since berthollides (the covalent nature of the bond prevails in the compounds)
3. Interaction with "non-oxidizing" acids (HCl, H 2 SO 4 dil.)
Fe 0 + 2H + → Fe 2+ + H 2
Since Fe is located in the activity series to the left of hydrogen (E ° Fe / Fe 2+ \u003d -0.44V), it is able to displace H 2 from ordinary acids.
Fe + 2HCl \u003d FeCl 2 + H 2
Fe + H 2 SO 4 \u003d FeSO 4 + H 2
4. Interaction with "oxidizing" acids (HNO 3 , H 2 SO 4 conc.)
Fe 0 - 3e - → Fe 3+
Concentrated HNO 3 and H 2 SO 4 "passivate" iron, so at ordinary temperatures the metal does not dissolve in them. With strong heating, slow dissolution occurs (without release of H 2).
In razb. HNO 3 iron dissolves, goes into solution in the form of Fe 3+ cations, and the acid anion is reduced to NO *:
Fe + 4HNO 3 \u003d Fe (NO 3) 3 + NO + 2H 2 O
It dissolves very well in a mixture of HCl and HNO 3
5. Attitude to alkalis
Fe does not dissolve in aqueous solutions of alkalis. It reacts with molten alkalis only at very high temperatures.
6. Interaction with salts of less active metals
Fe + CuSO 4 \u003d FeSO 4 + Cu
Fe 0 + Cu 2+ = Fe 2+ + Cu 0
7. Interaction with gaseous carbon monoxide (t = 200°C, P)
Fe (powder) + 5CO (g) \u003d Fe 0 (CO) 5 iron pentacarbonyl
Fe(III) compounds
Fe 2 O 3 - iron oxide (III).
Red-brown powder, n. R. in H 2 O. In nature - "red iron ore".
Ways to get:
1) decomposition of iron hydroxide (III)
2Fe(OH) 3 = Fe 2 O 3 + 3H 2 O
2) pyrite roasting
4FeS 2 + 11O 2 \u003d 8SO 2 + 2Fe 2 O 3
3) decomposition of nitrate
Chemical properties
Fe 2 O 3 is a basic oxide with signs of amphoterism.
I. The main properties are manifested in the ability to react with acids:
Fe 2 O 3 + 6H + = 2Fe 3+ + ZN 2 O
Fe 2 O 3 + 6HCI \u003d 2FeCI 3 + 3H 2 O
Fe 2 O 3 + 6HNO 3 \u003d 2Fe (NO 3) 3 + 3H 2 O
II. Weak acid properties. Fe 2 O 3 does not dissolve in aqueous solutions of alkalis, but when fused with solid oxides, alkalis and carbonates, ferrites are formed:
Fe 2 O 3 + CaO \u003d Ca (FeO 2) 2
Fe 2 O 3 + 2NaOH \u003d 2NaFeO 2 + H 2 O
Fe 2 O 3 + MgCO 3 \u003d Mg (FeO 2) 2 + CO 2
III. Fe 2 O 3 - feedstock for iron production in metallurgy:
Fe 2 O 3 + ZS \u003d 2Fe + ZSO or Fe 2 O 3 + ZSO \u003d 2Fe + ZSO 2
Fe (OH) 3 - iron (III) hydroxide
Ways to get:
Obtained by the action of alkalis on soluble salts Fe 3+:
FeCl 3 + 3NaOH \u003d Fe (OH) 3 + 3NaCl
At the time of receipt of Fe(OH) 3 - red-brown mucosamorphous precipitate.
Fe (III) hydroxide is also formed during the oxidation of Fe and Fe (OH) 2 in humid air:
4Fe + 6H 2 O + 3O 2 \u003d 4Fe (OH) 3
4Fe(OH) 2 + 2Н 2 O + O 2 = 4Fe(OH) 3
Fe(III) hydroxide is the end product of hydrolysis of Fe 3+ salts.
Chemical properties
Fe(OH) 3 is a very weak base (much weaker than Fe(OH) 2). Shows noticeable acidic properties. Thus, Fe (OH) 3 has an amphoteric character:
1) reactions with acids proceed easily:
2) a fresh precipitate of Fe(OH) 3 is dissolved in hot conc. solutions of KOH or NaOH with the formation of hydroxo complexes:
Fe (OH) 3 + 3KOH \u003d K 3
In an alkaline solution, Fe (OH) 3 can be oxidized to ferrates (salts of iron acid H 2 FeO 4 not isolated in the free state):
2Fe(OH) 3 + 10KOH + 3Br 2 = 2K 2 FeO 4 + 6KBr + 8H 2 O
Fe 3+ salts
The most practically important are: Fe 2 (SO 4) 3, FeCl 3, Fe (NO 3) 3, Fe (SCN) 3, K 3 4 - yellow blood salt \u003d Fe 4 3 Prussian blue (dark blue precipitate)
b) Fe 3+ + 3SCN - \u003d Fe (SCN) 3 Fe (III) thiocyanate (blood red solution)
The first products made of iron and its alloys were found during excavations and date back to about the 4th millennium BC. That is, even the ancient Egyptians and Sumerians used meteorite deposits of this substance to make jewelry and household items, as well as weapons.
Today, various kinds of iron compounds, as well as pure metal, are the most common and used substances. No wonder the 20th century was considered iron. Indeed, before the advent and widespread use of plastic and related materials, it was this compound that was of decisive importance for humans. What is this element and what substances it forms, we will consider in this article.
Chemical element iron
If we consider the structure of the atom, then first of all we should indicate its location in the periodic system.
The chemical element iron also has about 20 different isotopes that are not stable. The possible oxidation states that a given atom can exhibit are:
Not only the element itself is important, but also its various compounds and alloys.
Physical Properties
As a simple substance, iron has a pronounced metallicity. That is, it is a silvery-white metal with a gray tint, which has a high degree of ductility and ductility and a high melting and boiling point. If we consider the characteristics in more detail, then:
Depending on the conditions and different temperatures, there are several modifications that iron forms. Their physical properties differ from the fact that the crystal lattices differ.

All modifications have different types of structure of crystal lattices, and also differ in magnetic properties.
Chemical properties
As mentioned above, the simple substance iron exhibits medium chemical activity. However, in a finely dispersed state, it is capable of self-ignition in air, and the metal itself burns out in pure oxygen.
The corrosion ability is high, so the alloys of this substance are coated with alloying compounds. Iron is able to interact with:
It is obvious that, showing such activity, the metal is able to form various compounds, diverse and polar in properties. And so it happens. Iron and its compounds are extremely diverse and are used in various branches of science, technology, and industrial human activity.
Distribution in nature
Natural iron compounds are quite common, because it is the second most common element on our planet after aluminum. At the same time, in its pure form, the metal is extremely rare, as part of meteorites, which indicates its large accumulations in space. The main mass is contained in the composition of ores, rocks and minerals.

If we talk about the percentage of the element in question in nature, then the following figures can be given.
The most common iron compounds form the following minerals:
This is still a long list, because there are really a lot of them. In addition, various alloys that are created by man are widespread. These are also such iron compounds, without which it is difficult to imagine the modern life of people. These include two main types:
Iron is also a valuable addition to many nickel alloys.
Iron(II) compounds
These include those in which the oxidation state of the forming element is +2. They are quite numerous, because they include:
The formulas of chemical compounds in which iron exhibits the indicated degree of oxidation are individual for each class. Consider the most important and common of them.
Length and Distance Converter Mass Converter Bulk Food and Food Volume Converter Area Converter Volume and Recipe Units Converter Temperature Converter Pressure, Stress, Young's Modulus Converter Energy and Work Converter Power Converter Force Converter Time Converter Linear Velocity Converter Flat Angle Converter thermal efficiency and fuel efficiency Converter of numbers in different number systems Converter of units of measurement of quantity of information Currency rates Dimensions of women's clothing and shoes Dimensions of men's clothing and shoes Angular velocity and rotational frequency converter Acceleration converter Angular acceleration converter Density converter Specific volume converter Moment of inertia converter Moment of force converter Torque converter Specific calorific value converter (by mass) Energy density and specific calorific value converter (by volume) Temperature difference converter Coefficient converter Thermal Expansion Coefficient Thermal Resistance Converter Thermal Conductivity Converter Specific Heat Capacity Converter Energy Exposure and Radiant Power Converter Heat Flux Density Converter Heat Transfer Coefficient Converter Volume Flow Converter Mass Flow Converter Molar Flow Converter Mass Flux Density Converter Molar Concentration Converter Mass Concentration in Solution Converter Dynamic ( Kinematic Viscosity Converter Surface Tension Converter Vapor Permeability Converter Water Vapor Flux Density Converter Sound Level Converter Microphone Sensitivity Converter Sound Pressure Level (SPL) Converter Sound Pressure Level Converter with Selectable Reference Pressure Brightness Converter Luminous Intensity Converter Illuminance Converter Computer Graphics Resolution Converter Frequency and wavelength converter Power in diopters and focal length Distance Power in Diopters and Lens Magnification (×) Electric Charge Converter Linear Charge Density Converter Surface Charge Density Converter Volumetric Charge Density Converter Electric Current Converter Linear Current Density Converter Surface Current Density Converter Electric Field Strength Converter Electrostatic Potential and Voltage Converter Electrical Resistance Converter Converter Electrical Resistance Electrical Conductivity Converter Electrical Conductivity Converter Capacitance Inductance Converter US Wire Gauge Converter Levels in dBm (dBm or dBm), dBV (dBV), watts, etc. units Magnetomotive force converter Magnetic field strength converter Magnetic flux converter Magnetic induction converter Radiation. Ionizing Radiation Absorbed Dose Rate Converter Radioactivity. Radioactive Decay Converter Radiation. Exposure Dose Converter Radiation. Absorbed Dose Converter Decimal Prefix Converter Data Transfer Typography and Image Processing Unit Converter Timber Volume Unit Converter Calculation of Molar Mass Periodic Table of Chemical Elements by D. I. Mendeleev
Chemical formula
Molar mass of Fe 2 (SO 4) 3 , iron (III) sulfate 399.8778 g/mol
55.845 2+(32.065+15.9994 4) 3
Mass fractions of elements in the compound
Using the Molar Mass Calculator
Molar mass calculator
mole
All substances are made up of atoms and molecules. In chemistry, it is important to accurately measure the mass of substances entering into a reaction and resulting from it. By definition, the mole is the SI unit for the amount of a substance. One mole contains exactly 6.02214076×10²³ elementary particles. This value is numerically equal to the Avogadro constant N A when expressed in units of moles⁻¹ and is called Avogadro's number. Amount of substance (symbol n) of a system is a measure of the number of structural elements. A structural element can be an atom, a molecule, an ion, an electron, or any particle or group of particles.
Avogadro's constant N A = 6.02214076×10²³ mol⁻¹. Avogadro's number is 6.02214076×10²³.
In other words, a mole is the amount of a substance equal in mass to the sum of the atomic masses of the atoms and molecules of the substance, multiplied by the Avogadro number. The mole is one of the seven basic units of the SI system and is denoted by the mole. Since the name of the unit and its symbol are the same, it should be noted that the symbol is not inflected, unlike the name of the unit, which can be declined according to the usual rules of the Russian language. One mole of pure carbon-12 equals exactly 12 grams.
Molar mass
Molar mass is a physical property of a substance, defined as the ratio of the mass of that substance to the amount of the substance in moles. In other words, it is the mass of one mole of a substance. In the SI system, the unit of molar mass is kilogram/mol (kg/mol). However, chemists are accustomed to using the more convenient unit g/mol.
molar mass = g/mol

Molar mass of elements and compounds
Compounds are substances made up of different atoms that are chemically bonded to each other. For example, the following substances, which can be found in the kitchen of any housewife, are chemical compounds:
The molar mass of chemical elements in grams per mole is numerically the same as the mass of the element's atoms, expressed in atomic mass units (or daltons). The molar mass of compounds is equal to the sum of the molar masses of the elements that make up the compound, taking into account the number of atoms in the compound. For example, the molar mass of water (H₂O) is approximately 1 × 2 + 16 = 18 g/mol.
Molecular mass

Molecular weight (the old name is molecular weight) is the mass of a molecule, calculated as the sum of the masses of each atom that makes up the molecule, multiplied by the number of atoms in this molecule. The molecular weight is dimensionless a physical quantity numerically equal to the molar mass. That is, the molecular weight differs from the molar mass in dimension. Although the molecular mass is a dimensionless quantity, it still has a value called the atomic mass unit (amu) or dalton (Da), and is approximately equal to the mass of one proton or neutron. The atomic mass unit is also numerically equal to 1 g/mol.
Molar mass calculation
The molar mass is calculated as follows:
For example, let's calculate the molar mass of acetic acid
It consists of:
Our calculator does just that. You can enter the formula of acetic acid into it and check what happens.
Do you find it difficult to translate units of measurement from one language to another? Colleagues are ready to help you. Post a question to TCTerms and within a few minutes you will receive an answer.