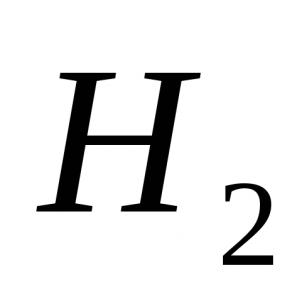How to determine the polarity of a bond? Direct and reverse polarity. Electronegativity
Rice. 32. Schemes of polar and non-polar molecules: a - polar molecule; b-non-polar molecule
In any molecule there are both positively charged particles - the nuclei of atoms, and negatively charged particles - electrons. For each kind of particles (or, rather, charges), one can find a point that will be, as it were, their "electric center of gravity." These points are called the poles of the molecule. If in a molecule the electrical centers of gravity of positive and negative charges coincide, the molecule will be non-polar. Such, for example, are H 2 and N 2 molecules formed by identical atoms, in which common pairs of electrons equally belong to both atoms, as well as many symmetrically constructed molecules with atomic bonds, for example, methane CH 4, CCl 4 tetrachloride.
But if the molecule is built asymmetrically, for example, it consists of two heterogeneous atoms, as we have already said, the common pair of electrons can be more or less shifted towardsone of the atoms. Obviously, in this case, due to the uneven distribution of positive and negative charges inside the molecule, their electrical centers of gravity will not coincide and a polar molecule will be obtained (Fig. 32).
Polar molecules are
Polar molecules are dipoles. This term denotes in general any electrically neutral system, i.e., a system consisting of positive and negative charges distributed in such a way that their electrical centers of gravity do not coincide.
The distance between the electric centers of gravity of those and other charges (between the poles of the dipole) is called the length of the dipole. The length of the dipole characterizes the degree of polarity of the molecule. It is clear that for different polar molecules the length of the dipole is different; the larger it is, the more pronounced the polarity of the molecule.
Rice. 33. Schemes of the structure of CO2 and CS2 molecules
In practice, the degree of polarity of certain molecules is determined by measuring the so-called dipole moment of the molecule m, which is defined as the product of the dipole length l on the charge of its pole e:
t =l e
The values of dipole moments are associated with certain properties of substances and can be determined experimentally. Order of magnitude t always 10 -18, since the electric charge
throne is 4.80 10 -10 electrostatic units, and the length of the dipole is a value of the same order as the diameter of the molecule, i.e. 10 -8 cm. Below are the dipole moments of the molecules of some inorganic substances.
Dipole moments of some substances
t 10 18
. . . .. …….. 0
Water……. 1.85
. . . ………..0
Hydrogen chloride……. 1.04
Carbon dioxide…….0
bromide. …… 0.79
Carbon disulfide…………0
Hydrogen iodide…….. 0.38
Hydrogen sulfide………..1.1
Carbon monoxide……. 0,11
Sulphur dioxide. . . ……1.6
Hydrocyanic acid……..2.1
Determining the values of dipole moments allows us to draw many interesting conclusions regarding the structure of various molecules. Let's look at some of these findings.

Rice. 34. Scheme of the structure of the water molecule
As expected, the dipole moments of hydrogen and nitrogen molecules are zero; molecules of these substancesare symmetrical and, therefore, the electric charges in them are distributed evenly. The absence of polarity in carbon dioxide and carbon disulfide shows that their molecules are also built symmetrically. The structure of the molecules of these substances is schematically shown in Fig. 33.
Somewhat unexpected is the presence of a rather large dipole moment near water. Since the formula for water is similar to the formulas for carbon dioxide
and carbon disulfide, one would expect that its molecules would be built in the same waysymmetrically, like the CS 2 and CO 2 molecules.
However, in view of the experimentally established polarity of water molecules (polarity of molecules), this assumption has to be discarded. At present, an asymmetric structure is attributed to the water molecule (Fig. 34): two hydrogen atoms are connected to an oxygen atom in such a way that their bonds form an angle of about 105 °. A similar arrangement of atomic nuclei exists in other molecules of the same type (H 2 S, SO 2) that have dipole moments.
The polarity of water molecules explains many of its physical properties.
A molecule is polar if the center of the negative charge does not coincide with the center of the positive one. Such a molecule is a dipole: two charges of equal magnitude and opposite in sign are separated in space.
A dipole is usually denoted by the symbol where the arrow points from the positive end of the dipole to the negative. A molecule has a dipole moment, which is equal to the magnitude of the charge multiplied by the distance between the charge centers:
![]()
Dipole moments of molecules can be measured; some found values are given in table. 1.2. The values of dipole moments serve as a measure of the relative polarity of various molecules.
Table 1.2 (see scan) Dipole moments
There is no doubt that the molecules are polar, if only the bonds in it are polar. We will consider bond polarity because the polarity of a molecule can be thought of as the sum of the polarities of the individual bonds.
Molecules such as have a dipole moment equal to zero, that is, they are non-polar. Two identical atoms in any given molecule have, of course, the same electronegativity and equally own electrons; the charge is zero and therefore the dipole moment is also zero.
The type molecule has a large dipole moment Although the hydrogen fluoride molecule is small, the electronegative fluorine strongly attracts electrons; although the distance is small, the charge is large, and hence the dipole moment is also large.
Methane and carbon tetrachloride have zero dipole moments. Individual bonds, at least in carbon tetrachloride, are polar: however, due to the symmetry of the tetrahedral arrangement, they compensate each other (Fig. 1.9). In methyl chloride, the polarity of the carbon-chlorine bond is not compensated and the dipole moment of methyl chloride is. Thus, the polarity of the molecules depends not only on the polarity of the individual bonds, but also on their direction, i.e., on the shape of the molecule.
The dipole moment of ammonia is It can be considered as the total dipole moment (vector sum) of three moments of individual bonds having the direction shown in the figure.

Rice. 1.9. Dipole moments of some molecules. Polarity of bonds and molecules.
Similarly, we can consider the dipole moment of water equal to

What dipole moment should be expected for nitrogen trifluoride, which, like ammonia, has a pyramidal structure? Fluorine is the most electronegative element, and it certainly draws electrons strongly from nitrogen; therefore, the nitrogen-fluorine bonds must be strongly polar and their vector sum must be large - much more than for ammonia with its not very polar -bonds.

What gives the experiment? The dipole moment of nitrogen trifluoride is only He is much less than the dipole moment of ammonia.
How to explain this fact? In the above consideration, the lone pair of electrons was not taken into account. B (as well as in this pair occupies the -orbital and its contribution to the dipole moment should have the opposite direction compared to the total moment of the nitrogen-fluorine bonds (Fig. 1.10); these moments of the opposite sign, obviously, have approximately the same value, and as a result, there is a small dipole moment, the direction of which is unknown.In ammonia, the dipole moment is probably determined mainly by this free electron pair, and it is increased by the sum of the bond moments.Similarly, lone pairs of electrons should contribute to the dipole moments of water and, of course, any other molecules in which they are present.
Based on the values of dipole moments, valuable information about the structure of molecules can be obtained. For example, any structure of carbon tetrachloride that results in a polar molecule can be ruled out only on the basis of the magnitude of the dipole moment.

Rice. 1.10. Dipole moments of some molecules. The contribution of the lone pair of electrons. The dipole moment due to the lone pair of electrons has a direction opposite to the direction of the total vector of bond moments.
Thus, the dipole moment confirms the tetrahedral structure of carbon tetrachloride (although it does not, since other structures are possible that will also give a non-polar molecule).
Task 1.4. Which of the two possible structures listed below would also have to have a zero dipole moment? a) Carbon is located in the center of the square, at the corners of which there are chlorine atoms, b) Carbon is located at the top of the tetrahedral pyramid, and chlorine atoms are at the corners of the base.
Task 1.5. Although the carbon-oxygen and boron-fluorine bonds must be polar, the dipole moment of the compounds is zero. Propose an arrangement of atoms for each compound, causing a zero dipole moment.
For most compounds, the dipole moment has never been measured. The polarity of these compounds can be predicted from their structure. The polarity of the bonds is determined by the electronegativity of the atoms; if the angles between the bonds are known, then the polarity of the molecule can be determined, also taking into account unpaired pairs of electrons.

On the hydrogen atom +0.17, and on the chlorine atom -0.17.
The so-called effective charges on atoms are most often used as a quantitative measure of bond polarity.
The effective charge is defined as the difference between the charge of electrons located in some region of space near the nucleus and the charge of the nucleus. However, this measure has only a conditional and approximate [relative] meaning, since it is impossible to single out unambiguously a region in a molecule that belongs exclusively to a single atom, and in the case of several bonds, to a specific bond.
The presence of an effective charge can be indicated by the symbols of charges on atoms (for example, H δ+ - Cl δ− , where δ is some fraction of the elementary charge) O − = C 2 + = O − (\displaystyle (\stackrel (-)(\mbox(O)))=(\stackrel (2+)(\mbox(C)))=(\stackrel (-)( \mbox(O))))(O δ− =C 2δ+ =O δ−), H δ+ -O 2δ− -H δ+ .
Almost all chemical bonds, with the exception of bonds in diatomic homonuclear molecules, are polar to one degree or another. Covalent bonds are usually weakly polar. Ionic bonds are highly polar.
Encyclopedic YouTube
1 / 5
✪ Ionic, covalent and metallic bonds
✪ Types of chemical bonds. Part 1.
✪ Chemistry. Chemical bond. Covalent bond and its characteristics. Foxford Online Learning Center
✪ CHEMICAL BOND Polarity Length Covalent Hydrogen Ionic OGE USE CHEMISTRY 2017 Task 3
✪ Chemistry. Covalent chemical bond in organic compounds. Foxford Online Learning Center
Subtitles
Effective charge
The values of relative effective charges obtained by various methods (optical spectroscopy, NMR, also based on quantum chemical calculations), may diverge. However, the available values of δ indicate that the atoms in compounds of high charges do not have [corresponding to the absolute charge electron] and there are no purely ionic compounds.
Instantaneous and induced dipoles.
A molecule is a dynamic system in which there is a constant movement of electrons and oscillation of nuclei. Therefore, the distribution of charges in it cannot be strictly constant. For example, the Cl 2 molecule is classified as non-polar: the value of its electric moment of the dipole is zero. However, at each given moment there is a temporary shift of charges to one of the chlorine atoms: Cl δ+ → Cl δ− or Cl δ− ← Cl δ+ with the formation instantaneous microdipoles. Since such a displacement of charges to any of the atoms is equally probable, the average distribution of charges exactly corresponds to the average zero value of the dipole moment.
For polar molecules, the value of the dipole moment at any given moment of time is somewhat greater or somewhat less than its average value. The direction and magnitude of the instantaneous dipole are subject to continuous fluctuations in the constant moment of the dipole. Thus, any non-polar and polar molecule (and an atom in it) can be considered as a set of periodic, very rapidly changing in magnitude and direction instantaneous microdipoles.
In the space around the nuclei in comparison with the distribution of electron density in the neutral atoms forming this bond.
The so-called effective charges on atoms are used as a quantitative measure of bond polarity.
The effective charge is defined as the difference between the charge of electrons located in some region of space near the nucleus and the charge of the nucleus. However, this measure has only a conditional and approximate meaning, since it is impossible to single out unambiguously a region in a molecule that belongs exclusively to a single atom, and in the case of several bonds, to a specific bond.
The presence of an effective charge can be indicated by the symbols of the charges of atoms (for example, H + δ - Cl - δ, where δ is some fraction of the elementary charge).
Almost all chemical bonds, with the exception of bonds in diatomic homonuclear molecules, are polar to one degree or another. Covalent bonds are usually weakly polar. Ionic bonds are highly polar.
see also
Sources
Wikimedia Foundation. 2010 .
- polar arrow
- Polar expeditions
See what "Chemical bond polarity" is in other dictionaries:
Polarity of chemical bonds- a characteristic of a chemical bond (See Chemical bond), showing the redistribution of the electron density in space near the nuclei compared to the initial distribution of this density in the neutral atoms that form this bond. ... ...
Polarity- Wiktionary has an article called "polarity" Polarity (← lat. polaris ← ... Wikipedia
chemical bond- ... Wikipedia
Molecule- Scheme of covalent bonds between atoms in an oxygen molecule ... Wikipedia
Valence (chemical)- Valency (from lat. valentia ≈ strength), the ability of an atom to form chemical bonds. It is customary to consider the number of other atoms in a molecule with which a given atom forms bonds with a quantitative measure of V.. V. ≈ one of the fundamental concepts ... ... Great Soviet Encyclopedia
Valence- I Valence (from lat. valentia strength) the ability of an atom to form chemical bonds. It is customary to consider the number of other atoms in a molecule with which a given atom forms bonds with a quantitative measure of V.. V. one of the fundamental ... ... Great Soviet Encyclopedia
Octet rule- Bonds in carbon dioxide (CO2) all atoms are surrounded by 8 electrons according to the octet rule. Therefore, CO2 is a stable molecule. The octet rule (octet theory) was proposed by G. N. Lewis to explain the reasons ... ... Wikipedia
Structural chemistry- Structural chemistry section, a field of chemistry that studies the relationship of various physical and physico-chemical properties of various substances with their chemical structure and reactivity. Structural chemistry considers not only geometric ... ... Wikipedia
Electronegativity- (χ) a fundamental chemical property of an atom, a quantitative characteristic of the ability of an atom in a molecule to displace common electron pairs towards itself. The modern concept of the electronegativity of atoms was introduced by the American chemist L. Pauling. ... ... Wikipedia
isomerism- Not to be confused with isomerism of atomic nuclei. Isomerism (from other Greek ἴσος “equal”, and μέρος “share, part”) is a phenomenon consisting in the existence of chemical compounds (isomers) that are identical in composition and molecular weight, but ... ... Wikipedia
When a covalent bond is formed between dissimilar atoms, the bonding pair of electrons shifts towards the more electronegative atom. This leads to the polarization of molecules, so all diatomic molecules consisting of dissimilar elements turn out to be polar to some extent. In more complex molecules, the polarity also depends on the geometry of the molecule. For the appearance of polarity, it is necessary that the centers of distribution of positive and negative charges do not coincide.
In the CO 2 molecule, the carbon-oxygen bonds are polar, with a certain positive charge on the carbon atom, and the same negative charge on each of the oxygen atoms. Therefore, the center of positive charge is concentrated on the carbon atom. Since the oxygen atoms are located on the same straight line, but both sides of the carbon atom (linear molecule) are at equal distances, the positive charge is neutralized. Thus, despite the polarity of each bond in CO., the entire molecule as a whole is non-polar and the reason for this is
Rice. 434. Examples of the structure and polarity of a molecule is its linear structure. On the contrary, the S=C=0 molecule is polar, since the carbon-sulfur and carbon-oxygen bonds have different lengths and different polarities. On fig. 4.34 shows the structures and polarity of some molecules.
It follows from the above examples that if the atoms or groups of atoms attached to the central atom are the same or located symmetrically relative to it (linear, flat triangular, tetrahedral and other structures), then the molecule will be nonpolar. If unequal groups are attached to the central atom or if there is an asymmetric arrangement of groups, then the molecules are polar.
When considering polar bonds, the effective charge of atoms in a molecule is important. For example, in the HC1 molecule, the binding electron cloud is shifted towards the more electronegative chlorine atom, as a result of which the charge of the hydrogen nucleus is not compensated, and the electron density on the chlorine atom becomes excessive compared to the charge of its nucleus. Therefore, the hydrogen atom is positively polarized, and the chlorine atom is negatively polarized. The hydrogen atom has a positive charge, and the chlorine atom has a negative charge. This charge 8, called the effective charge, is usually established experimentally. So, for hydrogen 8 H \u003d +0.18, and for chlorine 5 C, \u003d -0.18 of the absolute charge of the electron, as a result, the bond in the HC1 molecule is 18% ionic (i.e., the degree of ionicity is 0.18 ).
Since the polarity of the bond depends on the degree of displacement of the bonding pair of electrons towards the more electronegative element, the following must be taken into account:
- a) electronegativity (EO) is not a strict physical quantity that can be determined directly experimentally;
- b) the value of electronegativity is not constant, but depends on the nature of the other atom with which this atom is bonded;
- c) the same atom in a given chemical bond can sometimes function both as an electropositive and as an electronegative one.
Experimental evidence suggests that relative electronegativities (RERs) can be assigned to elements, the use of which makes it possible to judge the degree of polarity of the bond between atoms in a molecule (see also paragraphs 3.6 and 4.3).
In a molecule consisting of two atoms, the greater the polarity of the covalent bond, the higher the RER of one of them, therefore, with an increase in the RER of the second element, the degree of ionicity of the compound increases.
To characterize the reactivity of molecules, not only the nature of the electron density distribution is important, but also the possibility of its change under the influence of an external influence. The measure of this change is the polarizability of the bond, i.e. its ability to become polar or even more polar. Bond polarization occurs both under the influence of an external electric field and under the influence of another molecule that is a reaction partner. The result of these influences may be the polarization of the bond, accompanied by its complete break. In this case, the binding pair of electrons remains at the more electronegative atom, which leads to the formation of opposite ions. This type of bond breaking is called teterolytic. For example:

In the above example of an asymmetric bond rupture, hydrogen is split off in the form of an H + -ion, and the binding pair of electrons remains with chlorine, so the latter is converted into an anion C1.
In addition to this type of bond rupture, a symmetrical bond rupture is also possible, when not ions are formed, but atoms and radicals. This type of bond breaking is called homolytic.