First law of thermodynamics. Internal energy, heat
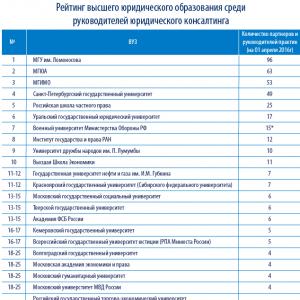
Physical processes such as heat and work can be explained by the simple transfer of energy from one body to another. In the case of work, we are talking about mechanical energy, while heat implies thermal energy. The transfer of energy is carried out according to the laws of thermodynamics. The main provisions of this section of physics are known as "beginnings".
The first law of thermodynamics regulates and limits the process of energy transfer in a particular system.
Types of energy systems
There are two types of energy systems in the physical world. A closed or closed system has a constant mass. In an open or open system, the mass can decrease and increase depending on the processes taking place in this system. Most of the observed systems are open.
Research in such systems is hampered by many random factors that affect the reliability of the results. Therefore, physicists study phenomena in closed systems, extrapolating the results to open ones, taking into account the necessary corrections.
Energy of an isolated system
Any closed system in which there is no exchange of energy with the environment is isolated. The equilibrium state of such a system is determined by the readings of the following quantities:
- P is the pressure in the system;
- V is the volume of the isolated system
- T- temperature;
- n is the number of moles of gas in the system;
as you can see, the amount of heat and the work done are not included in this list. A closed isolated system does not exchange heat and do no work. Its total energy remains unchanged.
Change in system energy
When work is performed or a heat transfer process occurs, the state of the system changes, and it will no longer be considered isolated.
Statement of the first law of thermodynamics
First of all, the first law of thermodynamics was derived for isolated systems. Later it was proved that the law is universal, and it can be applied to open systems, if one correctly takes into account the change in internal energy that occurs due to fluctuations in the amount of matter in the system. If the system under consideration passes from state A to state B, then the work done by the system W, and the amount of heat Q will differ. Different processes give different readings of these variables even if the system eventually returns to its original state. But at the same time, the difference W- Q will always be the same. In other words, if after any impact the system returned to its original state, then regardless of the type of processes involved in the transformation of such a system, the rule is observed W- Q= const.
In some cases, it is more convenient to use the differential formula for the expression of the first law. It looks like this: dU= dw- dQ
Here dU- infinitesimal change in internal energy
dW- quantity characterizing the infinitesimal work of the system
dQ- an infinitesimal amount of heat transferred to a given system.
Enthalpy
For a wider application of the first law of thermodynamics, the concept of enthalpy is introduced.
This is the name of the total amount of total energy of a substance and the product of volume and pressure. The physical expression of enthalpy can be represented by the following formula:
The absolute value of enthalpy is the sum of the enthalpies of all the parts that make up the system.

In quantitative terms, this value cannot be determined. Physicists operate only with the difference between the enthalpies of the final and initial states of the system. Indeed, in any calculation of the change in the state of the system, a certain level is chosen at which the potential energy is equal to zero. The same is true for calculating enthalpy. If we apply the concept of enthalpy, then the first law of thermodynamics for isoprocesses will look like this: dU= dw- dH
The enthalpy of any system depends on the internal structure of the substances that make up this system. These indicators, in turn, depend on the structure of the substance, its temperature, quantity and pressure. For complex substances, you can calculate the standard enthalpy of formation, which is equal to the amount of heat that is needed to form a mole of a substance from simple constituents. As a rule, the value of the standard enthalpy is negative, since heat is released in most cases during the synthesis of complex substances.
First law of thermodynamics in adiabatic processes
The application of the first law of thermodynamics for isoprocesses can be viewed graphically. For example, consider an adiabatic process in which the amount of heat remains constant throughout the entire time, that is Q= const. Such an isoprocess takes place in thermally insulated systems, or in such a short time that the system does not have time to exchange heat with the external environment. The slow expansion of a gas in a volume-pressure diagram is described by the following curve:

According to the graph, it is possible to justify the application of the first law of thermodynamics to isoprocesses. Since there is no change in the amount of heat in the adiabatic process, the change in internal energy is equal to the amount of work done. dU= - dW
It follows that the internal energy of the system decreases, and its temperature falls.
Examples of adiabatic processes
The converse statement is also true: a decrease in pressure in the absence of heat transfer sharply increases the temperature of the system. Approximately this is how gas expands in internal combustion engines. In Diesel engines, combustible gas is compressed 15 times. A short-term increase in temperature allows the combustible mixture to self-ignite.

We can consider another example of an adiabatic process - the free expansion of gases. To do this, consider the following installation, consisting of two containers:

In the first container there is gas, in the second it is absent. By turning the tap, we will ensure that the gas fills the entire volume allotted to it. If the system is sufficiently isolated, the gas temperature will remain unchanged. Since the gas did no work, the variable dW= const. It turned out that, other things being equal, the temperature of the gas decreases during expansion. The expansion of the gas occurs unevenly, so this process cannot be represented on the "pressure-volume" diagram.
The first law of thermodynamics is a universal law that applies to all observable processes in the universe. A deep understanding of the causes of certain energy transformations allows us to understand existing physical phenomena and discover new laws.
First law of thermodynamics
Plan
Internal energy.
Isoprocesses.
Works at isoprocesses.
adiabatic process.
Heat capacity.
internal energy of the body.
The internal energy of a body is composed of the kinetic energy of the translational and rotational motion of molecules, the kinetic and potential energy of the vibrational motion of atoms in molecules, the potential energy of interaction between molecules and intramolecular energy (intranuclear).
The kinetic and potential energy of the body as a whole is not included in the internal energy.
The internal energy of a thermodynamic system of bodies is composed of the internal energy of interaction between bodies and the internal energy of each body.
The work of a thermodynamic system on external bodies consists in changing the state of these bodies and is determined by the amount of energy that the thermodynamic system transfers to external bodies.
Heat is the amount of energy presented by the system to external bodies during heat exchange. Work and heat are not functions of the state of the system, but a function of the transition from one state to another.
Thermodynamic system - they call such a system, a set of macroscopic bodies that can exchange energy with each other and with the external environment (with other bodies) (For example, a liquid and a vapor above it). The thermodynamic system is characterized by the following parameters:
P, V, T, ρ etc.
The states of the system, when at least one of the parameters changes, are called non-equilibrium.
Thermodynamic systems that do not exchange energy with external bodies are called closed.
Thermodynamic process is the transition of a system from one state (P 1 , V 1 , T 1 ) to another (P 2 , V 2 , T 2 ) is an imbalance in the system.
First law of thermodynamics.
The amount of heat communicated to the system is used to increase the internal energy of the system and to perform work on external bodies by the system.
The first law of thermodynamics is a special case of the law of conservation of energy that takes into account the internal energy of the system:
Q= U 2 - U 1 + A;
U 1, U 2 - the initial and final values of the internal energy of the body.
Ais the work done by the system.
Q- The amount of heat reported to the system.
In differential form:
d Q= dU+ d A;
dU- there is a total differential, and it depends on the difference between the initial and final states of the system.
d QAndd A- incomplete differentials, depend on the process itself, that is, on the path of the process. Work is done when the volume changes:
d A= fdx= pSdx = pdV;
d A= pdV;
The first law of thermodynamics - a perpetual motion machine of the first kind is impossible, that is, an engine that would do work in a larger amount than the energy it receives from outside.

- does not depend on the integration path.


- depends on the integration path of the process function and cannot be written:
A 2 - A 1 ; Q 2 - Q 1 ;
A, Qare not state functions. It is impossible to speak about the law of work and heat.
This is nothing but the law of conservation of energy.
Isoprocesses.
1) Isochoric process:
V=Withonst;
The process of heating a gas in a closed volume.
d Q=dU+pdV,
pdv=0; d U=dU,
The first law of thermodynamics takes on this form.
Heat capacity atV- const:

The heat capacity is determined by the ratio of the increase in heat received by the system to the increase in temperature.

2) Isobaric process:
P= const;
d Q= dU+ d A;
Divide bydT(for 1 mole of gas):


 pV=RT,
pV=RT,

cp= CV+ R,
3) Isothermal process:
T= const,
P V = A;
Since the internal energy depends onT, then with isothermal expansiondU=0:
d Q= d A,
The heat supplied to the gas during isothermal expansion is entirely converted into the work of expansion.

dQtends to ∞,dTtends to 0.
4) Adiabatic process:
No heat exchange with the environment. The first law of thermodynamics takes the form:
d Q=0; dU+d A=0,
dU+d A=0; d A=-dU,
In an adiabatic process, work is done only due to the loss of internal energy of the gas.

Processes in whichd Q=0 - adiabatic. Adiabatic processes are always accompanied by a change in body temperature. Since during adiabatic expansion, work is done due to internal energy (1cal \u003d 4.19 J).
Work with isoprocesses.
1) Isochoric process:
V= const
d A= pdV=0; A v =0,
The work of pressure forces in an equilibrium process is numerically equal to the area under the curve depicting the process onPV- diagram:
d A= pdV.
2) Isobaric process:
p=const;
d A=pdV;

3) Isothermal process:
T= const;
d A=
pdV;


dV= RT;
 ;
;
Process equilibrium:

4) Adiabatic process:
d Q= dU+ pdV;
dU=-pdV,
d Q=0; dU=C v dT,
 ,
,


We integrate:

+ (γ-1) lnV= const,
(TV γ-1 )= const,
(TV γ-1 ) = const -the equationPoisson

;

RV γ = const.
6. Heat capacity.
1) The heat capacity of a body is the amount of heat that must be imparted to the body so that it heats up by 1 0 WITH.

C p = C V + R; C P > C V,

Heat capacity can be attributed to a unit of mass, one mole and a unit of volume. Accordingly: specific, molar, volumetric ([J / kg * deg]; [J / mol * deg]; [J / m 3* deg]).
2) Heat capacity in real gases:
Mole internal energy:
N a k= R,
 is the heat capacity of one mole at a constant volume (v=
const).
is the heat capacity of one mole at a constant volume (v=
const).
;
– heat capacity of one mole at constant pressure (p= const).
Specific heat.
 [
]
;
[
]
;
State function.
W= U+ PV; C p > C v

When heated while maintaining the P partQgoes for expansion. Only by expanding can R.
Isotherm:PV= const;
Adiabat:PV γ = const;

PV γ
Since γ>1, then the adiabatic curve goes steeper than the isotherm.
 ;
;
C v dT + pdV=0;
d A=pdV=-C v dT;



PV γ =P 1 V 1 γ ,



There are two forms of energy transfer from one body to another - this is the work of some bodies on others and the transfer of heat. The energy of mechanical motion can be converted into the energy of thermal motion and vice versa. In such energy transitions, the law of conservation of energy is fulfilled. When applied to the processes considered in thermodynamics, the law of conservation of energy is called the first law (or first law) of thermodynamics. This law is a generalization of empirical data.
Statement of the first law of thermodynamics
The first law of thermodynamics is formulated as follows:
The amount of heat that is supplied to the system is spent on the performance of work by this system (against external forces) and the change in its internal energy. In mathematical form, the first law of thermodynamics can be written in integral form:
where is the amount of heat received by the thermodynamic system; - change in the internal energy of the system under consideration; A is the work that the system performs on external bodies (against external forces).
In differential form, the first law of thermodynamics is written as:
where is the element of the amount of heat that the system receives; - infinitesimal work performed by a thermodynamic system; is an elementary change in the internal energy of the system under consideration. It should be noted that in formula (2) - an elementary change in internal energy is a total differential, in contrast to and .
The amount of heat is considered positive if the system receives heat and negative if heat is removed from the thermodynamic system. The work will be greater than zero if it is performed by the system, and the work will be considered negative if it is performed on the system by external forces.
In the event that the system returned to its original state, then the change in its internal energy will be equal to zero:
In this case, in accordance with the first law of thermodynamics, we have:
Expression (4) means that a perpetual motion machine of the first kind is impossible. That is, it is fundamentally impossible to create a periodically operating system (heat engine) that performs work that would be greater than the amount of heat received by the system from the outside. The statement about the impossibility of a perpetual motion machine of the first kind is also one of the options for formulating the first law of thermodynamics.
Examples of problem solving
EXAMPLE 1
| Exercise | How much heat () is transferred to an ideal gas having a volume V in the process of isochoric heating if its pressure changes by ? Consider that the number of degrees of freedom of a gas molecule is equal to i. |
| Solution | The basis for solving the problem is the first law of thermodynamics, which we will use in integral form: Since, according to the condition of the problem, the process with gas is isochoric (), then the work in this process is zero, then the first law of thermodynamics for the isochoric process will take the form: The change in internal energy is determined using the formula:
where i is the number of degrees of freedom of a gas molecule; - amount of substance; R is the universal gas constant. Since we do not know how the gas temperature changes in the process under consideration, we use the Mendeleev-Clapeyron equation to find: Let us express the temperature from (1.4), write the formulas for the two states of the system under consideration: Using expressions (1.5) we find : From expressions (1.3) and (1.6) it follows that for an isochoric process the change in internal energy can be found as: And from the first law of thermodynamics for our process (at ), we have that:
|
| Answer |
EXAMPLE 2
| Exercise | Find the change in the internal energy of oxygen (), the work done by it (A) and the amount of heat received () in the process (1-2-3), which is indicated on the graph (Fig. 1). Consider that m 3; 100 kPa; m 3; kPa. |
| Solution | The change in internal energy does not depend on the course of the process, since internal energy is a state function. It depends only on the final and initial states of the system. Therefore, we can write that the change in internal energy in the process 1-2-3 is:
where i is the number of degrees of freedom of the oxygen molecule (since the molecule consists of two atoms, we consider ), is the amount of substance, . The temperature difference can be found by using the ideal gas equation of state and looking at the process graph: |
For systems in which creatures, thermal processes (absorption or release of heat) are important. According to the first law of thermodynamics, thermodynamic system (eg, steam in a heat engine) can only do work due to its internal. energy or k.-l. ext. source of energy. The first law of thermodynamics is often formulated as the impossibility of the existence of a perpetual motion machine of the first kind, which would do work without drawing energy from some source.
P The first law of thermodynamics introduces the idea of the internal energy of a system as a function of state. When the system is informed of a certain amount of heat Q, the internal changes. energy of the system DU and the system does work A:
DU = Q + A.
P The first law of thermodynamics states that each state of the system is characterized by a certain value of internal. energy U, regardless of how the system is brought to a given state. Unlike the values of U, the values of A and Q depend on the process that led to the change in the state of the system. If the initial and final states a and b are infinitely close (transitions between such states are called infinitesimal processes), the first law of thermodynamics is written as:
This means that an infinitesimal change in ext. energy dU is the total differential of the state function,those. integral \u003d U b - U a, while infinitesimal quantities of heat and work are not differential. values, i.e. the integrals of these infinitesimal quantities depend on the chosen transition path between the states a and b (sometimes they are called incomplete differentials).
From the total number of work produced by the system of volume Y, one can single out the work of a reversible isothermal. extensions under the action of external pressure p e , equal to p e V, and all other types of work, each of which can be represented as the product of a certain generalized force acting on the system from the environment by a generalized coordinate x i , changing under the influence of the corresponding generalized force. For an infinitesimal process
![]()
P The first law of thermodynamics allows you to calculate the max. work obtained with isothermal expansion of an ideal gas, isothermal. evaporation of liquid at post. pressure, establish the laws of adiabatic. expansion of gases, etc. The first law of thermodynamics is the basis of thermochemistry, which considers systems in which heat is absorbed or released as a result of chemical. p-tions, phase transformations. or dissolution (dilution solutions).
If the system exchanges with the environment not only energy, but also in-tion (see Open system), the change in ext. the energy of the system during the transition from the initial state to the final state includes, in addition to the work A and heat Q, also the so-called. mass energy Z. An infinitesimal amount of mass energy in an infinitesimal process is determined by chem. potentials m k of each of the components of the system:= , where dN k is an infinitesimal change in the number of moles of the k-th component as a result of exchange with the medium.
In the case of a quasi-static process, at Krom the system at each moment of time is in equilibrium with the environment, the first law of thermodynamics in general has a trace. mat. expression:

where p and m k are equal to the corresponding values for
First law of thermodynamics
The first law of thermodynamics is the law of conservation of energy, one of the universal laws of nature (along with the laws of conservation of momentum, charge and symmetry):
Energy is indestructible and uncreated; it can only change from one form to another in equivalent proportions.
The first law of thermodynamics is a postulate - it does not have to be proven logically or derived from any more general provisions. The truth of this postulate is confirmed by the fact that none of its consequences is in conflict with experience. Here are some more formulations of the first law of thermodynamics:
The total energy of an isolated system is constant;
A perpetual motion machine of the first kind (an engine that does work without expending energy) is impossible.
The first law of thermodynamics establishes the relationship between heat Q, work A and the change in the internal energy of the system ∆U:
The change in the internal energy of the system is equal to the amount of heat imparted to the system minus the amount of work done by the system against external forces.
∆U = Q-A (1.1)
dU = δQ-δA (1.2)
Equation (1.1) is a mathematical notation of the 1st law of thermodynamics for the finite, equation (1.2) - for an infinitely small change in the state of the system.
Internal energy is a state function; this means that the change in internal energy ∆U does not depend on the path of the system transition from state 1 to state 2 and is equal to the difference between the values of internal energy U 2 and U 1 in these states:
∆U \u003d U 2 -U 1 (1.3)
It should be noted that it is impossible to determine the absolute value of the internal energy of the system; thermodynamics is only interested in the change in internal energy during a process.
Consider the application of the first law of thermodynamics to determine the work performed by the system in various thermodynamic processes (we will consider the simplest case - the work of expansion of an ideal gas).
Isochoric process (V = const; ∆V = 0).
Since the work of expansion is equal to the product of pressure and volume change, for an isochoric process we get:
Isothermal process (T = const).
From the equation of state of one mole of an ideal gas, we obtain:
δA = PdV = RT(I.7)
Integrating expression (I.6) from V 1 to V 2 , we obtain
A=RT= RTln= RTln (1.8)
Isobaric process (P = const).
Qp = ∆U + P∆V (1.12)
In equation (1.12) we group variables with the same indices. We get:
Q p \u003d U 2 -U 1 + P (V 2 -V 1) \u003d (U 2 + PV 2) - (U 1 + PV 1) (1.13)
Let us introduce a new system state function - enthalpy H, which is identically equal to the sum of internal energy and the product of pressure and volume: H = U + PV. Then expression (1.13) is transformed to the following form:
Qp= H 2 -H 1 =∆H(1.14)
Thus, the thermal effect of an isobaric process is equal to the change in the enthalpy of the system.
Adiabatic process (Q= 0, δQ= 0).
In an adiabatic process, the expansion work is done by reducing the internal energy of the gas:
A = -dU=C v dT (1.15)
If Cv does not depend on temperature (which is true for many real gases), the work produced by the gas during its adiabatic expansion is directly proportional to the temperature difference:
A = -C V ∆T (1.16)
Task number 1. Find the change in internal energy during the evaporation of 20 g of ethanol at its boiling point. The specific heat of vaporization of ethyl alcohol at this temperature is 858.95 J/g, the specific vapor volume is 607 cm 3 /g (neglect the volume of liquid).
Solution:
1. Calculate the heat of vaporization of 20 g of ethanol: Q=q sp m=858.95J/g 20g = 17179J.
2. Calculate the work to change the volume of 20 g of alcohol during its transition from a liquid state to a vapor state: A= P∆V,
where P is the vapor pressure of alcohol ͵ equal to atmospheric, 101325 Pa (because any liquid boils when its vapor pressure is equal to atmospheric pressure).
∆V \u003d V 2 -V 1 \u003d V W -V p, because V<< V п, то объмом жидкости можно пренебречь и тогда V п =V уд ·m. Cледовательно, А=Р·V уд ·m. А=-101325Па·607·10 -6 м 3 /г·20г=-1230 Дж
3. Calculate the change in internal energy:
∆U \u003d 17179 J - 1230 J \u003d 15949 J.
Since ∆U>0, therefore, during the evaporation of ethanol, an increase in the internal energy of alcohol occurs.
The first law of thermodynamics - concept and types. Classification and features of the category "The first law of thermodynamics" 2017, 2018.
The properties of bodies during their mechanical and thermal interaction with each other can be described quite well on the basis of molecular-kinetic theory. According to this theory, all bodies are composed of the smallest particles - atoms, molecules or ions that are in ....
Internal energy can change mainly due to two processes: due to the work done on the system, and due to the communication of a certain amount of heat to the system. For example, the work changes when the piston moves, when external forces do work on the gas, ... .
. (2) Here by is meant the work done by the body. An infinitesimal change in the amount of heat, as well as, is not always a complete differential. According to the definition, internal energy is a single-valued function of the state of a thermodynamic system....
Thermal processes can be divided into two main types - quasi-static (quasi-equilibrium) and non-equilibrium. Quasi-static processes consist of continuously following one after another states of equilibrium. To describe such a process, you can use ....
Topic 1. Fundamentals of molecular physics and thermodynamics. Summary. All of these processes can be considered as special cases of a more complex process in which pressure and volume are related by an equation. (10) For n = 0 the equation describes an isobar, for n = 1 –... .
Equilibrium processes in an ideal gas. Heat capacity of an ideal gas. 4. Types of equilibrium processes. Definition 1. The internal energy of an object is a part of its total energy minus the kinetic energy of the object's motion, as ... .