Potassium permanganate alkane in an acidic medium. Oxidation of various classes of organic compounds
St. Petersburg State Technological Institute
(Technical University)
department organic chemistry Faculty 4
Group 476
Course work
Alkene oxidation
Student……………………………………… Rytina A.I.
Lecturer………………………………... Piterskaya Yu.L.
Saint Petersburg
Introduction
1. Epoxidation (reaction by N.A. Prilezhaev, 1909)
2. Hydroxylation
2.1anti-Hydroxylation
2.2syn-Hydroxylation
3. Oxidative cleavage of alkenes
4.Ozonolysis
5. Oxidation of alkenes in the presence of palladium salts
Conclusion
List of sources used
Introduction
Oxidation is one of the most important and widespread transformations of organic compounds.
In organic chemistry, oxidation is understood as processes that lead to the depletion of a compound in hydrogen or its enrichment in oxygen. In this case, electrons are removed from the molecule. Accordingly, reduction is understood as the detachment from an organic oxygen molecule or the addition of hydrogen to it.
In redox reactions, oxidizing agents are compounds with a high electron affinity (electrophiles), and reducing agents are compounds that have a tendency to donate electrons (nucleophiles). The ease of oxidation of the compound increases with the growth of its nucleophilicity.
During the oxidation of organic compounds, as a rule, complete transfer of electrons and, accordingly, a change in the valence of carbon atoms does not occur. Therefore, the concept of the degree of oxidation - the conditional charge of an atom in a molecule, calculated on the basis of the assumption that the molecule consists only of ions - is only conditional, formal.
When compiling the equations of redox reactions, it is necessary to determine the reducing agent, oxidizing agent and the number of given and received electrons. As a rule, the coefficients are selected using the electron-ion balance method (half-reaction method).
This method considers the transition of electrons from one atom or ion to another, taking into account the nature of the medium (acidic, alkaline or neutral) in which the reaction takes place. To equalize the number of oxygen and hydrogen atoms, either water molecules and protons (if the medium is acidic) or water molecules and hydroxide ions (if the medium is alkaline) are introduced.
Thus, when writing the reduction and oxidation half-reactions, one must proceed from the composition of the ions actually present in the solution. Substances that are poorly dissociated, poorly soluble or evolved as a gas should be written in molecular form.
As an example, consider the process of ethylene oxidation with a dilute aqueous solution of potassium permanganate (Wagner reaction). During this reaction, ethylene is oxidized to ethylene glycol, and potassium permanganate is reduced to manganese dioxide. Two hydroxyls 1 are added at the place of the double bond:
3C 2 H 4 + 2KMnO 4 + 4H 2 O → 3C 2 H 6 O 2 + 2MnO 2 + 2KOH
Reduction half-reaction: MnO 4 ¯ + 2H 2 O + 3 e → MnO 2 + 4OH ¯ 2
Oxidation half-reaction: C 2 H 4 + 2OH − − 2 e → C 2 H 6 O 2 3
Finally, we have in ionic form:
2MnO 4 ¯ + 4H 2 O + 3C 2 H 4 + 6OH ¯ → 2MnO 2 + 8OH ¯ + 3C 2 H 6 O 2
After carrying out the necessary reductions of similar terms, we write the equation in molecular form:
3C 2 H 4 + 2KMnO 4 + 4 H 2 O \u003d 3C 2 H 6 O 2 + 2MnO 2 + 2KOH.
Characteristics of some oxidizing agents
Oxygen
Air oxygen is widely used in technological processes, as it is the cheapest oxidizing agent. But oxidation with air oxygen is fraught with difficulties associated with the control of the process, which proceeds in various directions. Oxidation is usually carried out at high temperature in the presence of catalysts.
Ozone
Ozone O 3 is used to obtain aldehydes and ketones, if it is difficult to obtain them in other ways. Most often, ozone is used to establish the structure of unsaturated compounds. Ozone is produced by the action of a quiet electrical discharge on oxygen. One of the significant advantages of ozonation, compared with chlorination, is the absence of toxins after treatment 2 .
Potassium permanganate
Potassium permanganate is the most commonly used oxidizing agent. The reagent is soluble in water (6.0% at 20ºC), as well as in methanol, acetone and acetic acid. For oxidation, aqueous (sometimes acetone) solutions of KMnO 4 are used in a neutral, acidic or alkaline medium. When carrying out the process in a neutral environment, salts of magnesium, aluminum are added to the reaction mass or carbon dioxide is passed through to neutralize the potassium hydroxide released during the reaction. The oxidation reaction of KMnO 4 in an acidic environment is most often carried out in the presence of sulfuric acid. The alkaline environment during oxidation is created by the KOH formed during the reaction, or it is initially added to the reaction mass. In slightly alkaline and neutral media, KMnO 4 oxidizes according to the equation:
KMnO4+ 3 e+ 2H 2 O \u003d K + + MnO 2 + 4OH ¯
in an acidic environment:
KMnO4+ 5 e+ 8H + = K + + Mn 2+ + 4H 2 O 3
Potassium permanganate is used to obtain 1,2-diols from alkenes, in the oxidation of primary alcohols, aldehydes and alkylarenes to carboxylic acids, and also for the oxidative cleavage of the carbon skeleton at multiple bonds.
In practice, a fairly large excess (more than 100%) of KMnO 4 is usually used. This is due to the fact that under normal conditions KMnO 4 partially decomposes into manganese dioxide with the release of O 2 . Explosively decomposes with concentrated H 2 SO 4 when heated in the presence of reducing agents; mixtures of potassium permanganate with organic substances are also explosive 4 .
Peracids
Peracetic and performic acids are obtained by reacting 25-90% hydrogen peroxide with the corresponding carboxylic acid according to the following reaction:
RCOOH + H 2 O 2 \u003d RCOOOH + H 2 O
In the case of acetic acid, this equilibrium is established relatively slowly, and sulfuric acid is usually added as a catalyst to accelerate the formation of peracid. Formic acid is strong enough on its own to provide a quick equilibrium.
Pertrifluoroacetic acid, obtained in a mixture with trifluoroacetic acid by the reaction of trifluoroacetic anhydride with 90% hydrogen peroxide, is an even stronger oxidizing agent. Similarly, peracetic acid can be obtained from acetic anhydride and hydrogen peroxide.
Solid m-chloroperbenzoic acid, because it is relatively safe to handle, quite stable and can be stored for a long time.
Oxidation occurs due to the released oxygen atom:
RCOOOH = RCOOH + [O]
Peracids are used to obtain epoxides from alkenes, as well as lactones from alicyclic ketones.
Hydrogen peroxide
Hydrogen peroxide is a colorless liquid, miscible with water, ethanol and diethyl ether. A 30% solution of H 2 O 2 is called perhydrol. A highly concentrated preparation may react explosively with organic substances. On storage, it decomposes into oxygen and water. The persistence of hydrogen peroxide increases with dilution. For oxidation, aqueous solutions of various concentrations (from 3 to 90%) are used in neutral, acidic or alkaline media.
H 2 O 2 \u003d H 2 O + [O]
By the action of this reagent on α,β-unsaturated carbonyl compounds in an alkaline medium, the corresponding epoxyaldehydes and ketones are obtained, peracids are synthesized by the oxidation of carboxylic acids in an acidic medium. A 30% solution of H 2 O 2 in acetic acid oxidizes alkenes to 1,2-diols. Hydrogen peroxide is used: to obtain organic and inorganic peroxides, Na perborate and percarbonate; as an oxidizing agent in rocket fuels; upon receipt of epoxides, hydroquinone, pyrocatechol, ethylene glycol, glycerin, vulcanization accelerators of the thiuram group, etc.; for bleaching oils, fats, fur, leather, textile materials, paper; for cleaning germanium and silicon semiconductor materials; as a disinfectant for the neutralization of domestic and industrial wastewater; in medicine; as a source of O 2 in submarines; H 2 O 2 is part of Fenton's reagent (Fe 2 + + H 2 O 2), which is used as a source of OH free radicals in organic synthesis 5 .
Ruthenium and osmium tetroxides
Osmium tetroxide OsO 4 is a white to pale yellow powder with mp. 40.6ºС; t. kip. 131.2ºС. Sublimates already at room temperature, soluble in water (7.47 g in 100 ml at 25ºС), СCl 4 (250 g in 100 g of solvent at 20ºС). In the presence of organic compounds, it turns black due to reduction to OsO 2 6 .
RuO 4 is a golden yellow prism with so pl. 25.4ºС, noticeably sublimates at room temperature. Sparingly soluble in water (2.03 g in 100 ml at 20ºС), very soluble in CCl 4 . A stronger oxidizing agent than OsO 4 . Above 100ºС explodes. Like osmium tetroxide, it has high toxicity and high cost.
These oxidizing agents are used for the oxidation of alkenes to α-glycols under mild conditions.
Dioxiranes
The most commonly used are dimethyldioxirane and methyl(trifluoromethyl)dioxirane.

Dioxiranes are most often obtained in situ from the corresponding ketones and KHSO 5 (or K 2 SO 4 KHSO 4 2KHSO 5 (oxone)) in a slightly alkaline medium 7:
Dioxiranes are distinguished by high reactivity combined with good selectivity and are used for the oxidation of non-activated C–H bonds in alkanes, in the preparation of epoxides from alkenes, for the oxidation of amines, oximes, sulfides, sulfoxides, etc.
The oxidation of alkenes can take place in several directions:
1) with the preservation of the carbon skeleton of the molecule; this is how epoxidation and hydroxylation of the double bond proceed, leading to the formation of vicinal trans- or cis-glycols.
2) with a break in the double bond; this is how ozonolysis and exhaustive oxidation of alkenes proceed, leading to the formation of various kinds of carbonyl compounds and carboxylic acids.
Depending on the type of oxidation, various oxidizing agents are used.
1. Epoxidation(reaction of N.A. Prilezhaev, 1909)
Acyclic and cyclic alkenes, when interacting with peracids (peracids) RCOOOH in a non-polar, indifferent medium, form epoxides (oxiranes), therefore the reaction itself is called the epoxidation reaction.

According to modern nomenclature IUPAC- a three-membered ring with one oxygen atom is called oxirane.
Epoxidation of alkenes should be considered as a synchronous, coordinated process, which does not involve ionic intermediates such as the OH+ hydroxyl cation. In other words, the epoxidation of alkenes is a process syn- addition of one oxygen atom to the double bond with the complete preservation of the configuration of the substituents at the double bond. 8

For epoxidation, a mechanism has been proposed that is characteristic of concerted processes.

Since the attack of the double bond by the oxygen atom of the peracid is equally probable on both sides of the plane of the double bond, the resulting oxiranes are either meso-forms, or mixtures of enantiomers. The following peracids are used as epoxidizing agents: perbenzoic, m-chlorperbenzoic, monoperphthalic, peracetic, trifluoroperacetic and performic. Aromatic peracids are used as individual reagents, while aliphatic peracids - CH 3 CO 3 H, CF 3 CO 3 H and HCO 3 H are not isolated into individual form, and are used after their formation in the interaction of 30% or 90% hydrogen peroxide and the corresponding carboxylic acid. Perbenzoic and m-chloroperbenzoic acid is obtained by oxidation of benzoic and m-chlorobenzoic acid with 70% hydrogen peroxide in a solution of methanesulfonic acid or from acid chlorides of these acids and hydrogen peroxide.


Monoperphthalic acid is obtained by a similar method from phthalic anhydride and 30% hydrogen peroxide.

At present, monoperoxyphthalic acid in the form of its Mg salt finds considerable application. This reagent can be used in mixtures of organic solvents with water 9 .
Initially, perbenzoic or monoperphthalic acids were used to obtain oxiranes (epoxides) 10:

Currently, epoxidation is most often used m-chloroperbenzoic acid. Unlike other peracids, it is stable during storage for a long time (up to 1 year) and is absolutely safe to handle. Yields of oxiranes obtained by oxidation of acyclic and cyclic alkenes m-chloroperbenzoic acid in a solution of methylene chloride, chloroform or dioxane are usually quite high 11 .



Peracids are often generated directly from a reaction mixture of 90% hydrogen peroxide and carboxylic acid in methylene chloride.

Alkenes with a double bond conjugated with a carbonyl group or another acceptor substituent are inactive and it is better to use stronger oxidizing agents for their oxidation, such as trifluoroperacetic acid obtained from trifluoroacetic anhydride and 90% hydrogen peroxide in methylene chloride. Peroxycarboximidic acids RC(NH)OOH are unstable intermediates formed in the reactions of nitriles with alkaline solutions of hydrogen peroxide 12:
The simplest oxirane, ethylene oxide, is produced industrially by the oxidation of ethylene with oxygen in the presence of silver as a catalyst 13 .

2. Hydroxylation
A number of oxidizing reagents are known, with the help of which, under mild conditions, it is possible to add two hydroxyl groups to alkenes.
The reaction of hydroxylation of alkenes, proceeding under the action of a cold solution of potassium permanganate and accompanied by its discoloration, is known as the Wagner reaction (1888). It currently has little synthetic use, since it is accompanied by the formation of a significant number of by-products. However, this reaction can be used in the study of the structure of an organic compound as a qualitative test for a double bond. Hydroxylation of cyclohexene by the action of an aqueous solution of potassium permanganate in the cold was first carried out by V.V. Markovnikov. At present, osmium(VIII) oxide is most often used for the hydroxylation of alkenes (Kriege reaction, 1936) 14 .
Osmium tetroxide OsO 4 is a colorless crystalline substance, melting at 40 o C and easily soluble in ether. During the oxidation of cyclohexene with an ether solution of this reagent, a black precipitate is formed, which is a cyclic ester of osmic acid I 15:
The ether is formed as a result of the simultaneous opening of the carbon-carbon double bond of the alkene and the two double bonds of the metal oxide. This ester is then hydrolyzed using sodium sulfite as a catalyst. The hydrolysis product is cis-cyclohexanediol-1,2(II), in which the hydroxyls are located in the β-region, i.e. in front, and hydrogens in the α-region, i.e. behind.
2.1 anti-Hydroxylation
The three-membered ring of oxiranes is easily opened under the action of a wide variety of nucleophilic reagents. The hydrolysis of oxiranes is catalyzed by both acids and bases. In both cases, vicinal diols, i.e., glycols, are formed. During acid catalysis, in the first stage, the protonation of the oxygen atom of oxirane occurs with the formation of a cyclic oxonium cation, which opens as a result of the nucleophilic attack of a water molecule 16:

The key step in ring opening, which determines the rate of the entire process, is the nucleophilic attack of water on the protonated form of oxirane. From the point of view of the mechanism, this process is similar to the opening of the bromonium ion during the nucleophilic attack of the bromide ion or another nucleophilic agent. From these positions, the stereochemical result should be the formation trance-glycols in the cleavage of cyclic epoxides. Indeed, during the acid-catalyzed hydrolysis of cyclohexene oxide or cyclopentene oxide, exclusively trance-1,2-diols.
Thus, the two-stage process of alkene epoxidation followed by acid hydrolysis of the epoxide corresponds in total to the reaction anti-hydroxylation of alkenes.
Both stages anti-hydroxylation of alkenes can be combined if the alkene is treated with aqueous 30-70% hydrogen peroxide in formic or trifluoroacetic acid. Both of these acids are strong enough to open the oxirane ring.

Opening of the oxirane ring, catalyzed by the base, also leads to the formation of cyclic trance-glycols.
Therefore, the two-stage process of epoxidation of alkenes followed by alkaline hydrolysis of epoxides is also a reaction anti-hydroxylation of alkenes.
2.2 syn-Hydroxylation
Some salts and oxides of transition metals in higher oxidation states are effective reagents. syn-hydroxylation of the double bond of an alkene, when both hydroxyl groups are attached to the same side of the double bond. Oxidation of alkenes with potassium permanganate is one of the oldest methods syn-double bond hydroxylation - continues to be widely used despite its inherent limitations. cis-1,2-cyclohexanediol was first obtained by V.V. Markovnikov in 1878 by hydroxylation of cyclohexene with an aqueous solution of potassium permanganate at 0 0 C.

This method was further developed in the works of the Russian scientist E.E. Wagner, therefore syn-hydroxylation of alkenes under the action of an aqueous solution of potassium permanganate is called the Wagner reaction. Potassium permanganate is a strong oxidizing agent that can not only hydroxylate the double bond, but also cleave the resulting vicinal diol. In order to avoid further degradation of the glycols as much as possible, the reaction conditions must be carefully controlled. Glycol yields are usually low (30-60%). Best Results are achieved by hydroxylation of alkenes in a slightly alkaline medium (рН~8 9) at 0-5 0 С with a diluted 1% aqueous solution of KMnO 4 17 .


Initially, when alkenes are oxidized with potassium permanganate, a cyclic permanganic acid ester is formed, which is immediately hydrolyzed to a vicinal diol.

The cyclic ester of permanganic acid was not isolated as an intermediate, but its formation follows from experiments with labeled 18 O potassium permanganate: both oxygen atoms in glycol turn out to be labeled upon oxidation of the alkene KMn 18 O 4 . This means that both oxygen atoms are transferred from the oxidizing agent and not from the solvent - water, which is in good agreement with the proposed mechanism.
Another method syn-hydroxylation of alkenes under the action of osmium (VIII) oxide OsO 4 was proposed by R. Krige in 1936. Osmium tetroxide is a colorless, volatile, crystalline substance, readily soluble in ether, dioxane, pyridine, and other organic solvents. When osmium tetroxide reacts with alkenes in ether or dioxane, a black precipitate of the osmic acid cyclic ester is formed - osmate, which can be easily isolated individually. The addition of OsO 4 to the double bond is markedly accelerated in pyridine solution. The decomposition of osmates to vicinal glycols is achieved by the action of an aqueous solution of sodium hydrosulfite or hydrogen sulfide.

Product Outputs syn-hydroxylation of alkenes in this method is much higher than when using permanganate as an oxidizing agent. An important advantage of the Krige method is the absence of products of the oxidative cleavage of alkenes, which is characteristic of permanganate oxidation 18 .
 19
19

Osmium tetroxide is a very expensive and hard-to-get reagent, besides it is toxic. Therefore, osmium(VIII) oxide is used in the synthesis of small amounts of hard-to-reach substances in order to obtain the highest diol yield. In order to simplify syn-hydroxylation of alkenes under the action of OsO 4 a technique was developed that allows using only catalytic amounts of this reagent. Hydroxylation of alkenes is carried out using hydrogen peroxide in the presence of OsO 4, for example:

In conclusion, we can give the stereochemical relationship between the alkene cis- or trance-configuration and configuration of the resulting vicinal diol, which can be cis- or trance-isomer, erythro- or treo-form, meso- or D,L-shape depending on the substituents in the alkene 20:

Similar stereochemical relationships are observed in other reactions syn- or anti- multiple bond additions of hydrogen, hydrogen halides, water, halogens, boron hydrides, and other reagents.
3. Oxidative cleavage alkenes
During the oxidation of alkenes with an alkaline aqueous solution of potassium permanganate when heated or with a solution of KMnO 4 in aqueous sulfuric acid, as well as during the oxidation of alkenes with a solution of chromium (VI) oxide CrO 3 in acetic acid or potassium dichromate and sulfuric acid, the initially formed glycol undergoes oxidative degradation. The end result is the splitting of the carbon skeleton at the site of the double bond and the formation of ketones and/or carboxylic acids as end products, depending on the substituents on the double bond. If both carbon atoms at the double bond contain only one alkyl group, the final product of exhaustive oxidation will be a mixture of carboxylic acids, the tetrasubstituted alkene at the double bond is oxidized to two ketones. Monosubstituted alkenes with a terminal double bond are cleaved to carboxylic acid and carbon dioxide 21 .

 22
22
Due to the low yields of carboxylic acids and ketones, the reactions of exhaustive oxidation of alkenes in the classical version have not found wide application and were previously used mainly to determine the structure of the initial alkene from the products of destructive oxidation. Currently, the oxidation of alkenes (R-CH=CH-R and R-CH=CH 2) to carboxylic acids (RCOOH) with potassium permanganate or dichromate is carried out under conditions of phase transfer catalysis. The yields of carboxylic acids in this case exceed 90%.

4. Ozonolysis of alkenes
The reaction of alkenes with ozone is the most important method for the oxidative cleavage of alkenes at the double bond. For many decades, this reaction served as the main method for determining the structure of the initial hydrocarbon, and also found application in the synthesis of various carbonyl compounds. The reaction of alkene with ozone is carried out by passing a current of ~5% mixture of ozone and oxygen into a solution of alkene in methylene chloride or ethyl acetate at -80 0 -100 0 C. The end of the reaction is controlled by a test for free ozone with potassium iodide. The mechanism of this peculiar and complex reaction has been established mainly thanks to the work of Krige. The first product of the 1,3-dipolar cycloaddition to the double bond is the so-called molozonide (1,2,3-trioxolane). This product is unstable and further spontaneously decomposes with ring opening and the formation of normal ozonide (1,2,4-trioxolane) 23 as the final product.
 24
24
It is now generally accepted that the transformation of molozonide into ordinary ozonide occurs by the splitting-recombination mechanism. Mollozonide undergoes spontaneous opening of the unstable 1,2,3-trioxolane ring with the formation of a carbonyl compound and a bipolar ion, which then react with each other also according to the 1,3-dipolar cycloaddition scheme.

The given scheme of the rearrangement of molozonide into normal ozonide is confirmed by the fact that if another carbonyl compound is present as an "interceptor" of the bipolar ion in the reaction mixture before the complete formation of the ozonide, then the so-called "mixed ozonide" is formed. For example, in ozonization cis-stilbene in the presence of benzaldehyde labeled with the 18 O isotope, the label is part of the ether, and not the peroxide bridge of the ozonide:
This result is in good agreement with the formation of a mixed ozonide upon recombination of a bipolar ion with labeled benzaldehyde:

Ozonides are highly unstable compounds that decompose explosively. They are not isolated individually, but split under the action of a wide variety of regents. It is necessary to distinguish between reductive and oxidative cleavage. During hydrolysis, ozonides are slowly split into carbonyl compounds and hydrogen peroxide. Hydrogen peroxide oxidizes aldehydes to carboxylic acids. This is the so-called oxidative decomposition of ozonides:

Thus, during the oxidative decomposition of ozonides, carboxylic acids and (or) ketones depending on the structure of the starting alkene. Air oxygen, hydrogen peroxide, peracids or silver hydroxide can be used as oxidizing agents. Most often in synthetic practice, hydrogen peroxide in acetic or formic acid, as well as hydrogen peroxide in an alkaline medium, is used for this purpose.


In practice, the method of oxidative decomposition of ozonides is mainly used to obtain carboxylic acids.
More important is the reductive cleavage of ozonides. The most commonly used reducing agents are zinc and acetic acid, triphenylphosphine, or dimethyl sulfide. In this case, the end products of ozonolysis are aldehydes or ketones, depending on the structure of the starting alkene.


 25
25
From the above examples, it can be seen that a tetra-substituted alkene at a double bond forms two ketones during ozonolysis and subsequent reductive decomposition of the ozonide, while a tri-substituted alkene gives a ketone and an aldehyde. A disubstituted symmetrical alkene forms two aldehydes during ozonolysis, and alkenes with a terminal bond form an aldehyde and formaldehyde.
An interesting modification of ozonolysis is the method where sodium borohydride is used as the ozonide reducing agent. In this case, the final reaction products are primary or secondary alcohols formed during the reduction of aldehydes and xtones, respectively 26 .


Ozonolysis of alkenes is a complex, time-consuming and explosive process that requires the use of special equipment. For this reason, other methods have been developed for the oxidative cleavage of alkenes to carbonyl compounds and carboxylic acids, which successfully replace the ozonolysis reaction in synthetic practice.
One of the modern preparative methods for the oxidative destruction of alkenes was proposed in 1955 by R. Lemieux. This method is based on the hydroxylation of alkenes with potassium permanganate, followed by cleavage of vicinal glycol with sodium periodate NaIO 4 at pH ~ 7 8. Periodate itself does not interact with alkene. The products of this two-step oxidative cleavage are ketones or carboxylic acids, since aldehydes are also oxidized to carboxylic acids under these conditions. In the Lemieux method, the laborious problem of separating one of the reaction products, manganese dioxide, does not arise, since both dioxide and manganate are again oxidized with periodate to a permanganate ion. This allows only catalytic amounts of potassium permanganate to be used. Below are some typical examples of the oxidative cleavage of alkenes by the Lemieux method.

Citronellol, an alcohol that is part of rose oil, geranium and lemon oils, is oxidized with a mixture of potassium permanganate and sodium periodate in aqueous acetone at 5 10 0 C to 6-hydroxy-4-methylhexanecarboxylic acid with a quantitative yield.

Another variation of this method uses catalytic amounts of osmium tetroxide instead of potassium permanganate (Lemieux & Johnson 1956). A particular advantage of the combination of OsO 4 and NaIO 4 is that it allows the oxidation to be stopped at the aldehyde stage. Osmium tetroxide adds to the double bond of the alkene to form osmate, which is oxidized by sodium periodate to carbonyl compounds with the regeneration of osmium tetroxide.

Instead of osmium tetroxide, ruthenium tetroxide RuO 4 can also be used. Lemieux-Johnson oxidative degradation of alkenes leads to the same products as ozonolysis with reductive cleavage of ozonides.

In terms characteristic of modern organic chemistry, this means that the combination of OsO 4 -NaIO 4 is synthetic equivalent ozonolysis of alkenes followed by reductive cleavage. Similarly, the oxidation of alkenes with a mixture of permanganate and periodate is the synthetic equivalent of ozonolysis with oxidative degradation of ozonides.
Thus, the oxidation of alkenes is not only a set of preparative methods for obtaining alcohols, epoxides, diols, aldehydes, ketones, and carboxylic acids; it is also one of the possible ways to establish the structure of the starting alkene. So, according to the result of the oxidative degradation of the alkene, one can determine the position of the double bond in the molecule, while the stereochemical result syn- or anti- hydroxylation of an alkene makes it possible to draw a conclusion about its geometry.
5. Oxidation of alkenes in the presence of palladium salts
The oxidation of alkenes to carbonyl compounds using palladium chloride has been the subject of a significant amount of research, mainly due to the importance for industry of the reaction for producing acetaldehyde from ethylene (the Wacker process). During oxidation, palladium chloride is reduced to palladium, and such a process would be of limited interest were it not for the fact that expensive palladium chloride can be used in catalytic amounts in the presence of a second oxidant, most commonly copper(II) chloride, which oxidizes palladium to palladium. (II), while itself being reduced to copper(I). Reoxidation of copper(I) to copper(II) can be carried out with atmospheric oxygen, so that the overall process is very attractive as an industrial oxidation method 27 .
Ethylene is easily oxidized to acetaldehyde 28:
Suggested Mechanism 29:
The reaction takes place in an acidic medium, is not accompanied by a change in the number of carbon atoms in an ethylene molecule, and is currently the main source of acetaldehyde in industry.
Oxidation of ethylene homologues under the same conditions occurs at the least hydrogenated carbon atom of the double bond with the formation of ketones. In particular, when propene is oxidized, acetone is obtained, and when cyclohexene is oxidized, cyclohexanone is obtained.
Conclusion
The oxidation reaction is an important group of double bond reactions. In general, oxidation reactions occupy a special place in organic chemistry. When considering these reactions, it is necessary to take into account not only the nature of the organic compound being oxidized, but also the nature of the oxidizing agent, the presence or absence of a catalyst, the medium in which the reaction proceeds, etc.
Therefore, it is often necessary to memorize oxidizing agents and the conditions for their use in order to obtain the desired oxidation product. For example, the oxidation reaction of alkenes with a dilute solution of potassium permanganate leads to the formation of diols (glycols), while a concentrated solution of potassium permanganate destroys the alkene molecule at the double bond with the formation of oxygen-containing products. Thus, the same oxidizing agent in different environments gives different oxidation products.
Among the oxidation reactions, the ozonation reaction considered by us is considered the most important, which makes it possible to establish the structure of the initial alkene from the final products. In this paper, the main reactions of alkene oxidation and catalysts that are used in the course of oxidative processes were considered.
List of sources used:
1) Reutov O. A. Organic chemistry. In 4 parts. Ch. 1.-M.: BINOM. Knowledge Laboratory, 2004.-567p.
2) Remy G. Course of inorganic chemistry. T. 1. M.: Publishing House of Foreign Literature, 1963. - 920 p.
3) D.V. Kazakov, A.I. Voloshin, V.P. Kazakov, V.V. Shereschovets, N.N. Kabalnova. Chemistry and chemiluminescence of dioxiranes. Moscow: Nauka, 1999
4) Traven V.F . Organic Chemistry: Textbook for High Schools: In 2 volumes / VF Traven. - M .: ICC "Akademkniga", 2004. - T. 1
5) Haynes A. Methods of oxidation of organic compounds. Alkanes, alkenes, alkynes and arenes. M.: Mir, 1988.
6) Morrison R., Boyd R. "Organic Chemistry" M.: Mir, 1974
7) Shabarov Yu.S. "Organic chemistry" part 1 M.: Chemistry 1994
8) Petrov A.A., Balyan H.V., Troshchenko A.T. Organic chemistry: A textbook for universities. - 5th ed., Revised. And additional - St. Petersburg: "Ivan Fedorov", 2002.-624 p.
9) L. Feather, M. Fizer Organic Chemistry. Advanced Course. Volume 1-M., "Chemistry", 1969, 688 p.
10) Plotnikov V.F., St. Petersburg Yu.L. Alkenes. Tutorial.-SPb: SPbGTI(TU)-2003.-20 p.
11) Neiland O. Ya. Chapter II. Alkenes // Organic Chemistry: Proc. for chem. universities. - M .: "Higher School", 1990.
12) March D. Organic chemistry: In 4 volumes / Per. from English. - M.: Mir, 1987. - T. 4. 470 p.
13) Terney A. Modern organic chemistry: In 2 vols. - M .: Mir, 1981.
14) Hauptman Z., Grefe Yu., Remane H. "Organic Chemistry" M.: Khimiya, 1979
15) Dryuk V.G., Malinovsky M.S. "Course of organic chemistry" Kyiv: Vishcha school 1987
16) http://www.chemistry.ssu.samara.ru/chem2/u442.htm
17) http://www.xumuk.ru/
1 Petrov A.A., Balyan H.V., Troshchenko A.T. Organic chemistry: Textbook for universities, p.85
26 26 Traven Abstract >> Chemistry
CH2 + 3O2 2CO2 + 2H2O b) At oxidation alkenes dilute solution of potassium permanganate are formed ... on a double bond. c) When hard oxidation alkenes boiling solution of potassium permanganate in acidic ...
Theory of general chemistry with elements of teaching methodology
Cheat sheet >> ChemistryThe method of obtaining carbonyl compounds - oxidation alcohols. As an oxidizing agent, you can ... yield ~ 90%) 5. Oxidation alkenes. Aldehydes and ketones are oxidation hydrocarbons of the ethylene series ... are not hydrated. 7. Reactions oxidation. Aldehydes and ketones...
Oxiranes (epoxides)
Abstract >> Chemistry...) 3,4-epoxy-1-butene Methods for the preparation of oxiranes Oxidation alkenes peracids The most widely used method... 1,2-epoxycyclo-m-chlorobenzoic acid hexane acid Oxidation alkenes organic peracids is accompanied by the addition of oxygen ...
General information about alcohols. Polyols
Abstract >> Chemistry...) can be obtained oxidation alkenes potassium permanganate or tetrokisdom .... Describe the reaction mechanism Oxidation polyols Oxidation ethylene glycol proceeds differently, ... mechanisms: (m 21) Periodic oxidation glycerin leads to the formation of formaldehyde ...
This material can be difficult to master with self-study, due to the large amount of information, many nuances, all kinds of BUT and IF. Read carefully!
What exactly will be discussed?
In addition to complete oxidation (combustion), some classes of organic compounds are characterized by partial oxidation reactions, while they are converted into other classes.
There are specific oxidizing agents for each class: CuO (for alcohols), Cu (OH) 2 and OH (for aldehydes) and others.
But there are two classic oxidizing agents, which, so to speak, are universal for many classes.
This is potassium permanganate - KMnO 4. And potassium dichromate (dichromate) - K 2 Cr 2 O 7. These substances are strong oxidizing agents due to manganese in the +7 oxidation state, and chromium in the +6 oxidation state, respectively.
Reactions with these oxidizing agents are quite common, but nowhere is there a holistic guide on how to choose the products of such reactions.
In practice, there are a lot of factors that affect the course of the reaction (temperature, medium, concentration of reagents, etc.). Often a mixture of products is obtained. Therefore, it is almost impossible to predict the product that is formed.
But this is not good for the Unified State Examination: there you can’t write “maybe either this, or this, or otherwise, or a mixture of products.” There needs to be specifics.
The compilers of the assignments have invested a certain logic, a certain principle according to which a certain product should be written. Unfortunately, they did not share with anyone.
This question in most manuals is rather slippery bypassed: two or three reactions are given as an example.
I present in this article what can be called the results of a study-analysis of USE tasks. The logic and principles of compiling the oxidation reactions with permanganate and dichromate have been unraveled with fairly high accuracy (in accordance with the USE standards). About everything in order.
Determination of the degree of oxidation.
First, when dealing with redox reactions, there is always an oxidizing agent and a reducing agent.
The oxidizing agent is manganese in permanganate or chromium in dichromate, the reducing agent is atoms in the organic (namely, carbon atoms).
It is not enough to define the products, the reaction must be equalized. For equalization, the electronic balance method is traditionally used. To apply this method, it is necessary to determine the oxidation states of reducing agents and oxidizing agents before and after the reaction.
At inorganic substances we know the degree of oxidation from the 9th grade:
But in organic, probably, in the 9th grade they were not determined. Therefore, before learning how to write OVR in organic chemistry, you need to learn how to determine the degree of oxidation of carbon in organic substances. This is done a little differently than in inorganic chemistry.
Carbon has a maximum oxidation state of +4, a minimum of -4. And it can show any degree of oxidation of this interval: -4, -3, -2, -1, 0, +1, +2, +3, +4.
First you need to remember what an oxidation state is.
The oxidation state is the conditional charge that occurs on an atom, assuming that the electron pairs are shifted completely towards the more electronegative atom.
Therefore, the oxidation state is determined by the number of displaced electron pairs: if it is shifted to a given atom, then it acquires an excess minus (-) charge, if from an atom, then it acquires an excess plus (+) charge. In principle, this is the whole theory that you need to know to determine the oxidation state of a carbon atom.
To determine the degree of oxidation of a particular carbon atom in a compound, we need to consider EACH of its bonds and see in which direction the electron pair will shift and what excess charge (+ or -) will arise from this on the carbon atom.
Let's look at specific examples:
At carbon three hydrogen bonds. Carbon and hydrogen - which is more electronegative? Carbon, then, along these three bonds, the electron pair will shift towards carbon. Carbon takes one negative charge from each hydrogen: it turns out -3
The fourth bond is with chlorine. Carbon and chlorine - which is more electronegative? Chlorine, which means that over this bond, the electron pair will shift towards chlorine. Carbon has one positive +1 charge.
Then, you just need to add: -3 + 1 = -2. The oxidation state of this carbon atom is -2.

Let's determine the oxidation state of each carbon atom:

Carbon has three bonds to hydrogen. Carbon and hydrogen - which is more electronegative? Carbon, then, along these three bonds, the electron pair will shift towards carbon. Carbon takes one negative charge from each hydrogen: it turns out -3
And one more bond with another carbon. Carbon and other carbon - their electronegativity is equal, so there is no displacement of the electron pair (the bond is not polar). 

This atom has two bonds with one oxygen atom, and one more bond with another oxygen atom (as part of the OH group). More electronegative oxygen atoms in three bonds pull an electron pair from carbon, and carbon has a +3 charge.
By the fourth bond, carbon is connected to another carbon, as we have already said, the electron pair does not shift along this bond.


Carbon is bonded to hydrogen atoms by two bonds. Carbon, as more electronegative, pulls one pair of electrons for each bond with hydrogen, acquires a charge of -2.
A carbon double bond is linked to an oxygen atom. The more electronegative oxygen attracts one electron pair for each bond. Together, two electron pairs are pulled from carbon. Carbon acquires a +2 charge.
Together it turns out +2 -2 = 0.

Let's determine the oxidation state of this carbon atom:
A triple bond with a more electronegative nitrogen gives carbon a charge of +3; there is no displacement of the electron pair due to the bond with carbon.

Oxidation with permanganate.
What will happen to permanganate?
The redox reaction with permanganate can proceed in different environments (neutral, alkaline, acidic). And it depends on the medium how exactly the reaction will proceed, and what products are formed in this case.
Therefore, it can go in three directions:

Permanganate, being an oxidizing agent, is reduced. Here are the products of his recovery:
- acid environment.
The medium is acidified with sulfuric acid (H 2 SO 4). Manganese is reduced to the +2 oxidation state. And the recovery products will be:
KMnO 4 + H 2 SO 4 → MnSO 4 + K 2 SO 4 + H 2 O
- Alkaline environment.
To create an alkaline environment, a fairly concentrated alkali (KOH) is added. Manganese is reduced to an oxidation state of +6. Recovery Products
KMnO 4 + KOH → K 2 MnO 4 + H 2 O
- Neutral environment(and slightly alkaline).
In a neutral medium, in addition to permanganate, water also enters into the reaction (which we write on the left side of the equation), manganese will be reduced to +4 (MnO 2), the reduction products will be:
KMnO 4 + H 2 O → MnO 2 + KOH
And in a slightly alkaline environment (in the presence of a low concentration KOH solution):
KMnO 4 + KOH → MnO 2 + H 2 O

What will happen to organics?
The first thing to learn is that it all starts with alcohol! This is the initial stage of oxidation. The carbon to which the hydroxyl group is attached undergoes oxidation.
When oxidized, the carbon atom "acquires" a bond with oxygen. Therefore, when writing down the scheme of the oxidation reaction, they write [O] above the arrow:
primary alcohol oxidized first to an aldehyde, then to a carboxylic acid:

Oxidation secondary alcohol breaks in the second stage. Since the carbon is in the middle, a ketone is formed, not an aldehyde (the carbon atom in the ketone group can no longer physically form a bond with the hydroxyl group):

Ketones, tertiary alcohols And carboxylic acids no longer oxidized

The oxidation process is stepwise - as long as there is where to oxidize and there are all conditions for this - the reaction goes on. Everything ends up with a product that does not oxidize under given conditions: a tertiary alcohol, a ketone, or an acid.
It is worth noting the stages of methanol oxidation. First, it is oxidized to the corresponding aldehyde, then to the corresponding acid:

A feature of this product (formic acid) is that the carbon in the carboxyl group is bonded to hydrogen, and if you look closely, you can see that this is nothing more than an aldehyde group:

And the aldehyde group, as we found out earlier, is oxidized further to the carboxyl:

Did you recognize the resulting substance? Its gross formula is H 2 CO 3 . This carbonic acid, which breaks down into carbon dioxide and water:
H 2 CO 3 → H 2 O + CO 2
Therefore, methanol, formic aldehyde and formic acid(due to the aldehyde group) are oxidized to carbon dioxide.
mild oxidation.
Mild oxidation is oxidation without strong heating in a neutral or slightly alkaline medium (0 is written above the reaction ° or 20 °) .
It is important to remember that alcohols do not oxidize under mild conditions. Therefore, if they are formed, then oxidation stops on them. What substances will enter into a mild oxidation reaction?
- Containing a C=C double bond (Wagner reaction).
In this case, the π-bond breaks and "sits" on the released bonds along the hydroxyl group. It turns out dihydric alcohol:

Let's write the reaction of mild oxidation of ethylene (ethene). Let's write down the initial substances and predict the products. At the same time, we do not write H 2 O and KOH yet: they can appear both on the right side of the equation and on the left. And we immediately determine the oxidation states of the substances involved in the OVR:

Let's make an electronic balance (we mean that there are two or two carbon atoms of the reducing agent, they are oxidized separately):

Let's set the coefficients:

At the end, add the missing products (H 2 O and KOH). There is not enough potassium on the right - it means that the alkali will be on the right. We put a coefficient in front of it. There is not enough hydrogen on the left, so water is on the left. We put a coefficient in front of it:

Let's do the same with propylene (propene):


Cycloalkene is often slipped. Let him not confuse you. This is a regular hydrocarbon with a double bond:

Wherever this double bond is, the oxidation will proceed in the same way:

- containing an aldehyde group.
The aldehyde group is more reactive (more easily reacts) than the alcohol group. Therefore, the aldehyde will oxidize. Before acid:

Consider the example of acetaldehyde (ethanal). Let's write down the reactants and products and arrange the oxidation states. Let's make a balance and put the coefficients in front of the reducing agent and oxidizing agent:

In a neutral medium and slightly alkaline, the course of the reaction will be slightly different.
In a neutral environment, as we remember, we write water on the left side of the equation, and alkali on the right side of the equation (formed during the reaction):

In this case, in the same mixture, acid and alkali are nearby. Neutralization takes place.

They cannot exist side by side and react, salt is formed:
Moreover, if we look at the coefficients in the equation, we will understand that acids are 3 moles, and alkalis are 2 moles. 2 moles of alkali can only neutralize 2 moles of acid (2 moles of salt are formed). And one mole of acid remains. So the final equation will be:

In a slightly alkaline environment, alkali is in excess - it is added before the reaction, so all the acid is neutralized:

A similar situation arises in the oxidation of methanal. It, as we remember, is oxidized to carbon dioxide:

It must be borne in mind that carbon monoxide (IV) CO 2 is acidic. And will react with alkali. And since carbonic acid is dibasic, both an acid salt and an average salt can be formed. It depends on the ratio between alkali and carbon dioxide:
If alkali is related to carbon dioxide as 2:1, then there will be an average salt:
Or alkali can be significantly more (more than twice). If it is more than twice, then the remainder of the alkali will remain:
3KOH + CO 2 → K 2 CO 3 + H 2 O + KOH
This will occur in an alkaline environment (where there is an excess of alkali, since it was added to the reaction mixture before the reaction) or in a neutral environment, when a lot of alkali is formed.
But if alkali is related to carbon dioxide as 1:1, then there will be an acid salt:
KOH + CO 2 → KHCO 3
If there is more carbon dioxide than needed, then it remains in excess:
KOH + 2CO 2 → KHCO 3 + CO 2
This will be in a neutral environment if little alkali is formed.
We write down the starting substances, products, draw up a balance, put down the oxidation states in front of the oxidizing agent, reducing agent and the products that are formed from them:

In a neutral environment, an alkali (4KOH) will form on the right:

Now we need to understand what will be formed when three moles of CO 2 and four moles of alkali interact.
3CO 2 + 4KOH → 3KHCO 3 + KOH
KHCO 3 + KOH → K 2 CO 3 + H 2 O
So it turns out like this:
3CO 2 + 4KOH → 2KHCO 3 + K 2 CO 3 + H 2 O
Therefore, on the right side of the equation we write two moles of hydrocarbonate and one mole of carbonate:

And in a slightly alkaline environment, there are no such problems: due to the fact that there is an excess of alkali, an average salt will form:

The same will happen with the oxidation of oxalic acid aldehyde:

As in the previous example, a dibasic acid is formed, and according to the equation, 4 moles of alkali should be obtained (since 4 moles of permanganate).
In a neutral environment, again, all the alkali is not enough to completely neutralize all the acid.
Three moles of alkali go to form an acid salt, one mole of alkali remains:
3HOOC–COOH + 4KOH → 3KOOC–COOH + KOH
And this one mole of alkali goes into interaction with one mole of acid salt:
KOOC–COOH + KOH → KOOC–COOK + H2O
It turns out like this:
3HOOC–COOH + 4KOH → 2KOOC–COOH + KOOC–COOK + H2O
Final equation:

In a weakly alkaline medium, an average salt is formed due to an excess of alkali:

- containing a triple bondC≡ C.
Remember what happened during the mild oxidation of double bond compounds? If you do not remember, then scroll back - remember.
The π-bond breaks, attaches to the carbon atoms at the hydroxyl group. Here the same principle. Just remember that there are two pi bonds in a triple bond. First, this happens at the first π-bond:
Then on another π-bond:

A structure in which one carbon atom has two hydroxyl groups is extremely unstable. When something is unstable in chemistry, it tends to “fall off” something. Water falls off, like this:

This results in a carbonyl group.
Consider examples:
Ethine (acetylene). Consider the stages of oxidation of this substance:

Water splitting:




As in the previous example, in one reaction mixture, acid and alkali. Neutralization occurs - salt is formed. As can be seen from the coefficient in front of the alkali permanganate, there will be 8 moles, that is, it is quite enough to neutralize the acid. Final equation:

Consider the oxidation of butyne-2:

Water splitting:

No acid is formed here, so there is no need to fool around with neutralization.
Reaction equation:

These differences (between the oxidation of carbon at the edge and in the middle of the chain) are clearly demonstrated by the example of pentyn:

Water splitting:

It turns out a substance of an interesting structure:

The aldehyde group continues to oxidize:
Let's write down the starting materials, products, determine the degree of oxidation, draw up a balance, put down the coefficients in front of the oxidizing agent and reducing agent:

Alkali should form 2 mol (since the coefficient in front of permanganate is 2), therefore, all acid is neutralized:

Hard oxidation.
Hard oxidation is the oxidation sour, strongly alkaline environment. And also, in neutral (or slightly alkaline), but when heated.
In an acidic environment, they are also sometimes heated. But in order for hard oxidation to proceed not in an acidic environment, heating is a prerequisite.
What substances will undergo severe oxidation? (First, we will analyze only in an acidic environment - and then we will add the nuances that arise during oxidation in a strongly alkaline and neutral or slightly alkaline (when heated) environment).
With hard oxidation, the process goes to the maximum. As long as there is something to oxidize, oxidation continues.
- Alcohols. Aldehydes.
Consider the oxidation of ethanol. Gradually, it oxidizes to an acid:

We write down the equation. We write down the starting substances, OVR products, put down the oxidation states, draw up a balance. Equalize the reaction:

If the reaction is carried out at the boiling point of the aldehyde, when it is formed, it will evaporate (fly away) from the reaction mixture without having time to oxidize further. The same effect can be achieved under very gentle conditions (low heat). In this case, we write aldehyde as a product:

Consider the oxidation of secondary alcohol using the example of propanol-2. As already mentioned, the oxidation terminates at the second stage (the formation of a carbonyl compound). Since a ketone is formed, which is not oxidized. Reaction equation:

Consider the oxidation of aldehydes in terms of ethanal. It also oxidizes to acid:

Reaction equation:

Methanal and methanol, as mentioned earlier, are oxidized to carbon dioxide:

Metanal:

- Containing multiple bonds.
In this case, the chain breaks along the multiple bond. And the atoms that formed it undergo oxidation (acquire a bond with oxygen). Oxidize as much as possible.
When a double bond is broken, carbonyl compounds are formed from fragments (in the scheme below: from one fragment - aldehyde, from the other - ketone)

![]()
Let's analyze the oxidation of pentene-2:

Oxidation of "scraps":

It turns out that two acids are formed. Write down the starting materials and products. Let's determine the oxidation states of the atoms that change it, draw up a balance, equalize the reaction: 
When compiling the electronic balance, we mean that there are two or two carbon atoms of the reducing agent, they are oxidized separately:

Acid will not always form. Consider, for example, the oxidation of 2-methylbutene:

Reaction equation:

Absolutely the same principle in the oxidation of compounds with a triple bond (only oxidation occurs immediately with the formation of an acid, without the intermediate formation of an aldehyde):

Reaction equation:

When a multiple bond is located exactly in the middle, then not two products are obtained, but one. Since the "scraps" are the same and they are oxidized to the same products:

Reaction equation:

- Double corona acid.
There is one acid in which carboxyl groups (crowns) are connected to each other:
This is oxalic acid. Two crowns side by side are difficult to get along. It is certainly stable under normal conditions. But due to the fact that it has two carboxyl groups connected to each other, it is less stable than other carboxylic acids.
And therefore, under especially harsh conditions, it can be oxidized. There is a break in the connection between the "two crowns":

Reaction equation:

- Benzene homologues (and their derivatives).
Benzene itself does not oxidize, due to the fact that aromaticity makes this structure very stable. 
But its homologues are oxidized. In this case, the circuit also breaks, the main thing is to know exactly where. Some principles apply:
- The benzene ring itself is not destroyed, and remains intact until the end, the bond is broken in the radical.
- The atom directly bonded to the benzene ring is oxidized. If after it the carbon chain in the radical continues, then the gap will be after it.


Let's analyze the oxidation of methylbenzene. There, one carbon atom in the radical is oxidized: 
Reaction equation:

Let's analyze the oxidation of isobutylbenzene:

Reaction equation:

Let's analyze the oxidation of sec-butylbenzene:

Reaction equation:

During the oxidation of benzene homologues (and derivatives of homologues) with several radicals, two-three- and more basic aromatic acids are formed. For example, the oxidation of 1,2-dimethylbenzene:

Derivatives of benzene homologues (in which the benzene ring has non-hydrocarbon radicals) are oxidized in the same way. Another functional group on the benzene ring does not interfere:


Subtotal. Algorithm "how to write down the reaction of hard oxidation with permanganate in an acidic environment":
- Write down the starting materials (organics + KMnO 4 + H 2 SO 4).
- Write down the products of organic oxidation (compounds containing alcohol, aldehyde groups, multiple bonds, as well as benzene homologues will be oxidized).
- Record the permanganate reduction product (MnSO 4 + K 2 SO 4 + H 2 O).
- Determine the degree of oxidation in OVR participants. Draw up a balance. Put down the coefficients for the oxidizing agent and reducing agent, as well as for the substances that are formed from them.
- Then it is recommended to calculate how many sulfate anions are on the right side of the equation, in accordance with this, put the coefficient in front of sulfuric acid on the left.
- At the end, put the coefficient in front of the water.
Severe oxidation in a strongly alkaline medium and a neutral or slightly alkaline (when heated) medium.
These reactions are much less common. We can say that such reactions are exotic. And as befits any exotic reactions, these were the most controversial.
Hard oxidation is also hard in Africa, so organics are oxidized in the same way as in an acidic environment.
Separately, we will not analyze the reactions for each class, since the general principle has already been stated earlier. We will analyze only the nuances.
Strongly alkaline environment :
In a strongly alkaline environment, permanganate is reduced to an oxidation state of +6 (potassium manganate):
KMnO 4 + KOH → K 2 MnO 4 .
In a strongly alkaline environment, there is always an excess of alkali, therefore, complete neutralization will take place: if carbon dioxide is formed, there will be a carbonate, if an acid is formed, there will be a salt (if the acid is polybasic - an average salt).
For example, the oxidation of propene:

Ethylbenzene oxidation:

Slightly alkaline or neutral when heated :
Here, too, the possibility of neutralization must always be taken into account.
If oxidation proceeds in a neutral environment and an acidic compound (acid or carbon dioxide) is formed, then the resulting alkali will neutralize this acidic compound. But not always alkali is enough to completely neutralize the acid.
When aldehydes are oxidized, for example, it is not enough (oxidation will proceed in the same way as in mild conditions - the temperature will simply speed up the reaction). Therefore, both salt and acid are formed (roughly speaking, remaining in excess).
We discussed this when we discussed the mild oxidation of aldehydes.
Therefore, if you have acid in a neutral environment, you need to carefully see if it is enough to neutralize all the acid. Particular attention should be paid to the neutralization of polybasic acids.
In a weakly alkaline environment, due to a sufficient amount of alkali, only medium salts are formed, as there is an excess of alkali.
As a rule, alkali during oxidation in a neutral environment is quite enough. And the reaction equation that in a neutral, that in a slightly alkaline medium will be the same.
For example, consider the oxidation of ethylbenzene:


Alkali is enough to completely neutralize the resulting acid compounds, even excess will remain:

3 moles of alkali are consumed - 1 remains.
Final equation:

This reaction in a neutral and slightly alkaline medium will proceed in the same way (in a slightly alkaline medium there is no alkali on the left, but this does not mean that it does not exist, it simply does not enter into a reaction).
Redox reactions involving potassium dichromate (bichromate).
Bichromate does not have such a wide variety of organic oxidation reactions in the exam.
Oxidation with dichromate is usually carried out only in an acidic environment. At the same time, chromium is restored to +3. Recovery products:
The oxidation will be tough. The reaction will be very similar to permanganate oxidation. The same substances will be oxidized that are oxidized by permanganate in an acidic environment, the same products will be formed.
Let's take a look at some of the reactions.
Consider the oxidation of alcohol. If the oxidation is carried out at the boiling point of the aldehyde, then it will leave their reaction mixture without being oxidized:

Otherwise, the alcohol can be directly oxidized to an acid.
The aldehyde produced in the previous reaction can be "caught" and made to oxidize to an acid:

Oxidation of cyclohexanol. Cyclohexanol is a secondary alcohol, so a ketone is formed:

If it is difficult to determine the oxidation states of carbon atoms using this formula, you can write on the draft:

Reaction equation:

Consider the oxidation of cyclopentene.
The double bond breaks (the cycle opens), the atoms that formed it are oxidized to the maximum (in this case, to the carboxyl group):


Some features of oxidation in the USE with which we do not entirely agree.
Those "rules", principles and reactions that will be discussed in this section, we consider not entirely correct. They contradict not only the real state of affairs (chemistry as a science), but also the internal logic school curriculum and the USE in particular.
But nevertheless, we are forced to give this material in the form that the USE requires.
We are talking about HARD oxidation.
Remember how benzene homologues and their derivatives are oxidized under harsh conditions? All radicals are terminated - carboxyl groups are formed. Scraps are oxidized already "independently":

So, if suddenly a hydroxyl group, or a multiple bond, appears on the radical, you need to forget that there is a benzene ring there. The reaction will go ONLY along this functional group (or multiple bond).
The functional group and multiple bond is more important than the benzene ring.

Let's analyze the oxidation of each substance:
First substance:

It is necessary not to pay attention to the fact that there is a benzene ring. From the point of view of the exam, this is just secondary alcohol. Secondary alcohols are oxidized to ketones, and ketones are not further oxidized:

Let this substance be oxidized with dichromate:

Second substance:

This substance is oxidized, just as a compound with a double bond (we do not pay attention to the benzene ring):

Let it oxidize in neutral permanganate when heated:

The resulting alkali is enough to completely neutralize carbon dioxide:
2KOH + CO 2 → K 2 CO 3 + H 2 O
Final equation:

Oxidation of the third substance:

Let the oxidation proceed with potassium permanganate in an acidic medium:

Oxidation of the fourth substance:

Let it oxidize in a strongly alkaline environment. The reaction equation will be:

And finally, this is how vinylbenzene is oxidized:

And it oxidizes to benzoic acid, it must be borne in mind that, according to the logic of the Unified State Examination, it oxidizes this way not because it is a derivative of benzene. Because it contains a double bond.
Conclusion.
This is all you need to know about redox reactions involving permanganate and dichromate in organics.
Do not be surprised if, some of the points outlined in this article, you hear for the first time. As already mentioned, this topic is very extensive and controversial. And despite this, for some reason, very little attention is paid to it.
As you may have seen, two or three reactions do not explain all the patterns of these reactions. Here you need an integrated approach and a detailed explanation of all points. Unfortunately, in textbooks and on Internet resources, the topic is not fully disclosed, or not disclosed at all.
I tried to eliminate these shortcomings and shortcomings and consider this topic in its entirety, and not in part. I hope I succeeded.
Thank you for your attention, all the best to you! Success in development chemical science and passing exams!
As already mentioned, the oxidation of organic matter is the introduction of oxygen into its composition and (or) the elimination of hydrogen. Recovery is the reverse process (the introduction of hydrogen and the elimination of oxygen). Given the composition of alkanes (СnH2n+2), we can conclude that they are incapable of participating in reduction reactions, but they can participate in oxidation reactions.
Alkanes are compounds with low degrees of carbon oxidation, and depending on the reaction conditions, they can be oxidized to form various compounds.
At ordinary temperatures, alkanes do not react even with strong oxidizing agents (H2Cr2O7, KMnO4, etc.). When introduced into an open flame, alkanes burn. At the same time, in an excess of oxygen, they are completely oxidized to CO2, where carbon has the highest degree oxidation +4, and water. The combustion of hydrocarbons leads to the rupture of all C-C connections and C-H and is accompanied by the release of a large amount of heat (exothermic reaction).
It is generally accepted that the mechanism of alkane oxidation includes a radical chain process, since oxygen itself is not very reactive, in order to abstract a hydrogen atom from an alkane, a particle is needed that will initiate the formation of an alkyl radical that will react with oxygen, giving a peroxy radical. The peroxy radical can then abstract a hydrogen atom from another alkane molecule to form an alkyl hydroperoxide and a radical.

It is possible to oxidize alkanes with atmospheric oxygen at 100-150 ° C in the presence of a catalyst - manganese acetate, this reaction is used in industry. Oxidation occurs when an air current is blown through molten paraffin containing a manganese salt.
Because as a result of the reaction, a mixture of acids is formed, then they are separated from the unreacted paraffin by dissolving in aqueous alkali, and then neutralized with mineral acid.
Directly in industry, this method is used to obtain acetic acid from n-butane:
Alkene oxidation
Alkene oxidation reactions are divided into two groups: 1) reactions in which the carbon skeleton is preserved, 2) reactions of oxidative destruction of the carbon skeleton of the molecule along the double bond.
Oxidation reactions of alkenes with preservation of the carbon skeleton
1. Epoxidation (Prilezhaev reaction)
Acyclic and cyclic alkenes, when interacting with peracids in a non-polar medium, form epoxides (oxiranes).
Also, oxiranes can be obtained by oxidation of alkenes with hydroperoxides in the presence of molybdenum-, tungsten-, vanadium-containing catalysts:

The simplest oxirane, ethylene oxide, is produced industrially by the oxidation of ethylene with oxygen in the presence of silver or silver oxide as a catalyst.

2. anti-hydroxylation (hydrolysis of epoxides)
Acid (or alkaline) hydrolysis of epoxides leads to the opening of the oxide cycle with the formation of transdiols.

In the first stage, the protonation of the oxygen atom of the epoxide occurs with the formation of a cyclic oxonium cation, which opens as a result of the nucleophilic attack of the water molecule.
Base-catalyzed epoxy ring opening also leads to the formation of trans-glycols.

3. syn-hydroxylation
One of the oldest methods for the oxidation of alkenes is the Wagner reaction (oxidation with potassium permanganate). Initially, during oxidation, a cyclic permanganate ester is formed, which is hydrolyzed to a vicinal diol:

In addition to the Wagner reaction, there is another method for the syn-hydroxylation of alkenes under the action of osmium (VIII) oxide, which was proposed by Krige. Under the action of osmium tetroxide on an alkene in ether or dioxane, a black precipitate of the cyclic ester of osmic acid is formed - osmate. However, the addition of OsO4 to the multiple bond is markedly accelerated in pyridine. The resulting black precipitate of osmate is easily decomposed by the action of an aqueous solution of sodium hydrosulfite:

Potassium permanganate or osmium(VIII) oxide oxidize the alkene to cis-1,2-diol.
Oxidative cleavage of alkenes
The oxidative cleavage of alkenes includes reactions of their interaction with potassium permanganate in alkaline or sulfuric acid, as well as oxidation with a solution of chromium trioxide in acetic acid or potassium dichromate and sulfuric acid. The end result of such transformations is the splitting of the carbon skeleton at the site of the double bond and the formation of carboxylic acids or ketones.
Monosubstituted alkenes with a terminal double bond are cleaved to a carboxylic acid and carbon dioxide:

If both carbon atoms in the double bond contain only one alkyl group, then a mixture of carboxylic acids is formed:

But if an alkene tetrasubstituted with a double bond is a ketone:

The reaction of ozonolysis of alkenes has acquired a much greater preparative significance. For many decades, this reaction served as the main method for determining the structure of the starting alkene. This reaction is carried out by passing a current of an ozone solution in oxygen, an alkene solution in methylene chloride or ethyl acetate at -80 ... -100 ° C. The mechanism of this reaction was established by Krige:


Ozonides are unstable compounds that decompose with an explosion. There are two ways of decomposition of ozonides - oxidative and reductive.
During hydrolysis, ozonides are split into carbonyl compounds and hydrogen peroxide. Hydrogen peroxide oxidizes aldehydes to carboxylic acids - this is oxidative decomposition:

Much more important is the reductive splitting of ozonides. Ozonolysis products are aldehydes or ketones, depending on the structure of the starting alkene:
In addition to the above methods, there is another method proposed in 1955 by Lemieux:
In the Lemieux method, there are no time-consuming procedures for separating manganese dioxide, since dioxide and manganate are again oxidized by periodate to the permanganate ion. This allows only catalytic amounts of potassium permanganate to be used.
4.5. Alkene oxidation
It is advisable to divide the reactions of alkene oxidation into two large groups: reactions in which the carbon skeleton is preserved and reactions of oxidative destruction of the carbon skeleton of the molecule along the double bond. The first group of reactions includes epoxidation, as well as hydroxylation, leading to the formation of vicinal diols (glycols). In the case of cyclic alkenes, hydroxylation forms vicinal trance- or cis-diols. Another group includes ozonolysis and reactions of exhaustive oxidation of alkenes, leading to the formation of various kinds of carbonyl compounds and carboxylic acids.
4.5.a. Oxidation reactions of alkenes with preservation of the carbon skeleton
1. Epoxidation (reaction by N.A. Prilezhaev, 1909)
Acyclic and cyclic alkenes, when interacting with peracids (peracids) RCOOOH in a non-polar, indifferent medium, form epoxides (oxiranes), therefore the reaction itself is called the epoxidation reaction.
According to modern nomenclature IUPAC- a three-membered ring with one oxygen atom is called oxirane.
Epoxidation of alkenes should be considered as a synchronous, coordinated process, which does not involve ionic intermediates such as the OH+ hydroxyl cation. In other words, the epoxidation of alkenes is a process syn- addition of one oxygen atom to the double bond with the complete preservation of the configuration of the substituents at the double bond.
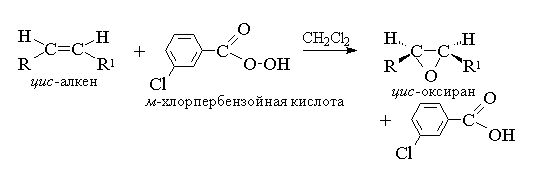

For epoxidation, a mechanism has been proposed that is characteristic of concerted processes.

Since the attack of the double bond by the oxygen atom of the peracid is equally probable on both sides of the plane of the double bond, the resulting oxiranes are either meso-forms, or mixtures of enantiomers. The following peracids are used as epoxidizing agents: perbenzoic, m-chlorperbenzoic, monoperphthalic, peracetic, trifluoroperacetic and performic. Aromatic peracids are used as individual reagents, while aliphatic peracids - CH 3 CO 3 H, CF 3 CO 3 H and HCO 3 H are not isolated individually, but are used after their formation in the interaction of 30% or 90% hydrogen peroxide and the corresponding carboxylic acid. Perbenzoic and m-chloroperbenzoic acid is obtained by oxidation of benzoic and m-chlorobenzoic acid with 70% hydrogen peroxide in a solution of methanesulfonic acid or from acid chlorides of these acids and hydrogen peroxide.


Monoperphthalic acid is obtained by a similar method from phthalic anhydride and 30% hydrogen peroxide.

Initially, perbenzoic or monoperphthalic acids were used to obtain oxiranes (epoxides):

Currently, epoxidation is most often used m-chloroperbenzoic acid. Unlike other peracids, it is stable during storage for a long time (up to 1 year) and is absolutely safe to handle. Yields of oxiranes obtained by oxidation of acyclic and cyclic alkenes m-chloroperbenzoic acid in a solution of methylene chloride, chloroform or dioxane are usually quite high.



Peracids are often generated directly from a reaction mixture of 90% hydrogen peroxide and carboxylic acid in methylene chloride.


Alkenes with a double bond conjugated with a carbonyl group or another acceptor substituent are inactive and it is better to use stronger oxidizing agents for their oxidation, such as trifluoroperacetic acid obtained from trifluoroacetic anhydride and 90% hydrogen peroxide in methylene chloride. The simplest oxirane, ethylene oxide, is produced industrially by the oxidation of ethylene with oxygen in the presence of silver as a catalyst.

2. anti-Hydroxylation
The three-membered ring of oxiranes is easily opened under the action of a wide variety of nucleophilic reagents. These reactions will be discussed in detail in the section on acyclic and cyclic ethers. Here, only the hydrolysis of oxiranes will be considered. The hydrolysis of oxiranes is catalyzed by both acids and bases. In both cases, vicinal diols, i.e., glycols, are formed. During acid catalysis, in the first stage, the protonation of the oxygen atom of oxirane occurs with the formation of a cyclic oxonium cation, which opens as a result of the nucleophilic attack of a water molecule:

The key step in ring opening, which determines the rate of the entire process, is the nucleophilic attack of water on the protonated form of oxirane. From the point of view of the mechanism, this process is similar to the opening of the bromonium ion during the nucleophilic attack of the bromide ion or another nucleophilic agent. From these positions, the stereochemical result should be the formation trance-glycols in the cleavage of cyclic epoxides. Indeed, during the acid-catalyzed hydrolysis of cyclohexene oxide or cyclopentene oxide, exclusively trance-1,2-diols.

Thus, the two-stage process of alkene epoxidation followed by acid hydrolysis of the epoxide corresponds in total to the reaction anti-hydroxylation of alkenes.
Both stages anti-hydroxylation of alkenes can be combined if the alkene is treated with aqueous 30-70% hydrogen peroxide in formic or trifluoroacetic acid. Both of these acids are strong enough to open the oxirane ring.

Opening of the oxirane ring, catalyzed by the base, also leads to the formation of cyclic trance-glycols.

Therefore, the two-stage process of epoxidation of alkenes followed by alkaline hydrolysis of epoxides is also a reaction anti-hydroxylation of alkenes.
3. syn-Hydroxylation
Some salts and oxides of transition metals in higher oxidation states are effective reagents. syn-hydroxylation of the double bond of an alkene, when both hydroxyl groups are attached to the same side of the double bond. Oxidation of alkenes with potassium permanganate is one of the oldest methods syn-double bond hydroxylation - continues to be widely used despite its inherent limitations. cis-1,2-cyclohexanediol was first obtained by V.V. Markovnikov in 1878 by hydroxylation of cyclohexene with an aqueous solution of potassium permanganate at 0 0 C.

This method was further developed in the works of the Russian scientist E.E. Wagner, therefore syn-hydroxylation of alkenes under the action of an aqueous solution of potassium permanganate is called the Wagner reaction. Potassium permanganate is a strong oxidizing agent that can not only hydroxylate the double bond, but also cleave the resulting vicinal diol. In order to avoid further degradation of the glycols as much as possible, the reaction conditions must be carefully controlled. Glycol yields are usually low (30-60%). The best results are achieved by hydroxylation of alkenes in a slightly alkaline medium (рН~8 9) at 0-5 0 С with a dilute 1% aqueous solution of KMnO 4 .


Initially, when alkenes are oxidized with potassium permanganate, a cyclic permanganic acid ester is formed, which is immediately hydrolyzed to a vicinal diol.

The cyclic ester of permanganic acid was not isolated as an intermediate, but its formation follows from experiments with labeled 18 O potassium permanganate: both oxygen atoms in glycol turn out to be labeled upon oxidation of the alkene KMn 18 O 4 . This means that both oxygen atoms are transferred from the oxidizing agent and not from the solvent - water, which is in good agreement with the proposed mechanism.
Another method syn-hydroxylation of alkenes under the action of osmium (VIII) oxide OsO 4 was proposed by R. Krige in 1936. Osmium tetroxide is a colorless, volatile, crystalline substance, readily soluble in ether, dioxane, pyridine, and other organic solvents. When osmium tetroxide reacts with alkenes in ether or dioxane, a black precipitate of the osmic acid cyclic ester is formed - osmate, which can be easily isolated individually. The addition of OsO 4 to the double bond is markedly accelerated in pyridine solution. The decomposition of osmates to vicinal glycols is achieved by the action of an aqueous solution of sodium hydrosulfite or hydrogen sulfide.

Product Outputs syn-hydroxylation of alkenes in this method is much higher than when using permanganate as an oxidizing agent. An important advantage of the Krige method is the absence of products of oxidative cleavage of alkenes, which is characteristic of permanganate oxidation.


Osmium tetroxide is a very expensive and hard-to-get reagent, besides it is toxic. Therefore, osmium(VIII) oxide is used in the synthesis of small amounts of hard-to-reach substances in order to obtain the highest diol yield. In order to simplify syn-hydroxylation of alkenes under the action of OsO 4 a technique was developed that allows using only catalytic amounts of this reagent. Hydroxylation of alkenes is carried out using hydrogen peroxide in the presence of OsO 4, for example:

To conclude this section, we present the stereochemical relationships between the alkene cis- or trance-configuration and configuration of the resulting vicinal diol, which can be cis- or trance-isomer, erythro- or treo-form, meso- or D,L-form depending on the substituents in the alkene:

Similar stereochemical relationships are observed in other reactions syn- or anti- multiple bond additions of hydrogen, hydrogen halides, water, halogens, boron hydrides, and other reagents.
Drawing up equations of redox reactions involving organic substances
IN connection with the introduction as the only form final certification alumni high school unified state exam(USE) and transition high school for specialized education, the preparation of high school students to perform the most “expensive” tasks of part “C” in terms of points is becoming increasingly important. USE test in chemistry. Despite the fact that the five tasks of part “C” are considered different: the chemical properties of inorganic substances, the chains of transformations of organic compounds, computational tasks, all of them are to some extent related to redox reactions (ORRs). If the basic knowledge of the OVR theory is mastered, then it is possible to correctly complete the first and second tasks in full, and the third - partially. In our opinion, a significant part of the success in the implementation of part "C" lies precisely in this. Experience shows that if, studying inorganic chemistry, students cope well enough with the tasks of writing OVR equations, then similar tasks in organic chemistry cause great difficulties for them. Therefore, throughout the study of the entire course of organic chemistry in specialized classes, we try to develop in high school students the skills of compiling OVR equations.
When studying comparative characteristics of inorganic and organic compounds, we introduce students to the use of the oxidation state (s.o.) (in organic chemistry, primarily carbon) and how to determine it:
1) calculation of the average s.d. carbon in a molecule of organic matter;
2) definition of s.d. every carbon atom.
We clarify in which cases it is better to use one or another method.
The article was published with the support of the company "GEO-Engineering", which presents products on the market under the brand name "ProfKresla". The company's field of activity is the production, sale and installation of armchairs and chairs for various halls. The high professionalism of employees and our own production facilities allow us to quickly and efficiently implement projects of any complexity. All products under the "ProfKresla" brand, whether they are theater chairs, seating for waiting rooms or chairs for educational institutions, are distinguished by a modern and ergonomic design, as well as high wear resistance, strength and comfort. From the huge range of products presented in the catalog on the profkresla.ru website, you can always choose models that best suit the corporate style adopted in your company. If you still have difficulties with the choice, then the company's specialists are always ready to give advice, help determine the model, and then prepare a project, make all the necessary measurements and installation on the spot.
P When studying the topic “Alkanes”, we show that the processes of oxidation, combustion, halogenation, nitration, dehydrogenation, and decomposition are redox processes. When writing the equations for the reactions of combustion and decomposition of organic substances, it is better to use the average value of s.d. carbon. For example:
We pay attention to the first half of the electronic balance: at the carbon atom in the fractional value of s.d. the denominator is 4, so we calculate the transfer of electrons using this coefficient.
In other cases, when studying the topic “Alkanes”, we determine the values of s.d. each carbon atom in the compound, while drawing students' attention to the sequence of substitution of hydrogen atoms at primary, secondary, tertiary carbon atoms:


Thus, we bring students to the conclusion that at the beginning the process of substitution takes place at the tertiary, then at the secondary, and, last of all, at the primary carbon atoms.
P When studying the topic “Alkenes”, we consider oxidation processes depending on the structure of the alkene and the reaction medium.
When alkenes are oxidized with a concentrated solution of potassium permanganate KMnO 4 in an acidic medium (hard oxidation), - and - bonds break with the formation of carboxylic acids, ketones and carbon monoxide (IV). This reaction is used to determine the position of the double bond.
If the double bond is at the end of the molecule (for example, in butene-1), then one of the oxidation products is formic acid, which is easily oxidized to carbon dioxide and water:

We emphasize that if in the alkene molecule the carbon atom at the double bond contains two carbon substituents (for example, in the molecule of 2-methylbutene-2), then during its oxidation a ketone is formed, since the transformation of such an atom into an atom of the carboxyl group is impossible without breaking C–C bond, relatively stable under these conditions:

We clarify that if the alkene molecule is symmetrical and the double bond is contained in the middle of the molecule, then only one acid is formed during oxidation:

We report that a feature of the oxidation of alkenes, in which the carbon atoms in the double bond contain two carbon radicals, is the formation of two ketones:


Considering the oxidation of alkenes in neutral or slightly alkaline media, we focus the attention of high school students on the fact that under such conditions, oxidation is accompanied by the formation of diols (dihydric alcohols), and hydroxyl groups are attached to those carbon atoms between which there was a double bond:

IN In a similar way, we consider the oxidation of acetylene and its homologues, depending on the medium in which the process takes place. So, we clarify that in an acidic environment, the oxidation process is accompanied by the formation of carboxylic acids:

The reaction is used to determine the structure of alkynes by oxidation products:

In neutral and slightly alkaline media, the oxidation of acetylene is accompanied by the formation of the corresponding oxalates (salts of oxalic acid), and the oxidation of homologues is accompanied by the breaking of the triple bond and the formation of salts of carboxylic acids:

IN All rules are worked out with students on specific examples, which leads to a better assimilation of theoretical material. Therefore, when studying the oxidation of arenes in various media, students can independently make assumptions that in an acidic medium one should expect the formation of acids, and in an alkaline medium, salts. The teacher will only have to clarify which reaction products are formed depending on the structure of the corresponding arena.
We show by examples that benzene homologues with one side chain (regardless of its length) are oxidized by a strong oxidizing agent to benzoic acid at the -carbon atom. Benzene homologues, when heated, are oxidized by potassium permanganate in a neutral medium to form potassium salts of aromatic acids.
5C 6 H 5 -CH 3 + 6KMnO 4 + 9H 2 SO 4 \u003d 5C 6 H 5 COOH + 6MnSO 4 + 3K 2 SO 4 + 14H 2 O,
5C 6 H 5 -C 2 H 5 + 12KMnO 4 + 18H 2 SO 4 \u003d 5C 6 H 5 COOH + 5CO 2 + 12MnSO 4 + 6K 2 SO 4 + 28H 2 O,
C 6 H 5 -CH 3 + 2KMnO 4 \u003d C 6 H 5 COOK + 2MnO 2 + KOH + H 2 O.
We emphasize that if there are several side chains in an arene molecule, then in an acidic medium each of them is oxidized at an a-carbon atom to a carboxyl group, resulting in the formation of polybasic aromatic acids:

P The acquired skills in compiling OVR equations for hydrocarbons allow them to be used in the study of the “Oxygen-containing compounds” section.
So, when studying the topic “Alcohols”, students independently compose the equations for the oxidation of alcohols, using the following rules:
1) primary alcohols are oxidized to aldehydes
3CH 3 -CH 2 OH + K 2 Cr 2 O 7 + 4H 2 SO 4 \u003d 3CH 3 -CHO + K 2 SO 4 + Cr 2 (SO 4) 3 + 7H 2 O;
2) secondary alcohols are oxidized to ketones

3) for tertiary alcohols, the oxidation reaction is not typical.
In order to prepare for USE teacher it is advisable to give additional information to these properties, which will undoubtedly be useful for students.
When methanol is oxidized with an acidified solution of potassium permanganate or potassium dichromate, CO 2 is formed, primary alcohols during oxidation, depending on the reaction conditions, can form not only aldehydes, but also acids. For example, the oxidation of ethanol with potassium dichromate in the cold ends with the formation of acetic acid, and when heated, acetaldehyde:
3CH 3 -CH 2 OH + 2K 2 Cr 2 O 7 + 8H 2 SO 4 \u003d 3CH 3 -COOH + 2K 2 SO 4 + 2Cr 2 (SO 4) 3 + 11H 2 O,
3CH 3 -CH 2 OH + K 2 Cr 2 O 7 + 4H 2 SO 4 3CH 3 -CHO + K 2 SO 4 + Cr 2 (SO 4) 3 + 7H 2 O.
Let us remind students again about the influence of the environment on the products of alcohol oxidation reactions, namely: a hot neutral solution of KMnO 4 oxidizes methanol to potassium carbonate, and the remaining alcohols to salts of the corresponding carboxylic acids:

When studying the topic “Aldehydes and ketones”, we focus students' attention on the fact that aldehydes are more easily oxidized than alcohols into the corresponding carboxylic acids not only under the action of strong oxidizing agents (air oxygen, acidified solutions of KMnO 4 and K 2 Cr 2 O 7), but and under the influence of weak (ammonia solution of silver oxide or copper (II) hydroxide):
5CH 3 -CHO + 2KMnO 4 + 3H 2 SO 4 \u003d 5CH 3 -COOH + 2MnSO 4 + K 2 SO 4 + 3H 2 O,
3CH 3 -CHO + K 2 Cr 2 O 7 + 4H 2 SO 4 \u003d 3CH 3 -COOH + Cr 2 (SO 4) 3 + K 2 SO 4 + 4H 2 O,
CH 3 -CHO + 2OH CH 3 -COONH 4 + 2Ag + 3NH 3 + H 2 O.
We pay special attention to the oxidation of methanal with an ammonia solution of silver oxide, since in this case, ammonium carbonate is formed, and not formic acid:
HCHO + 4OH \u003d (NH 4) 2 CO 3 + 4Ag + 6NH 3 + 2H 2 O.
As our long-term experience shows, the proposed method for teaching high school students how to write OVR equations with the participation of organic substances increases their final USE result in chemistry for a few points.







