Obtaining bromide butyl.
I.G. Bolesov, G.S. Zaitseva
Nucleophilic substitution and elimination.
Methodical development
Control N4
1. Write an equation for each of the following substitution reactions:
2. Write the haloalkanes and nucleophilic reagents required for the synthesis of the following products:

3. Draw each of the equations below using formulas that clearly indicate the stereochemistry of the reactants and products:
3.1(S)-2-bromobutane + MeONa (in methanol) 2-methoxybutane (S N 2),
3.2(R)-3-bromo-3-methylhexane + methanol 3-methoxy-3-methylhexane (S N 1),
3.3cis- 2-bromo-1-methylcyclopentane + NaSH 2-methylcyclopentanethiol.
4. Determine the order of reactivity for 1-bromo-2-methylpropane, bromide tert-butyl, 2-bromobutane in reactions with:
4.1. NaN 3 in dimethylformamide,
4.2. 10% aqueous dioxane.
5. Explain the gradual loss of optical activity by (R)-2-iodooctane when its solution in acetone is treated with sodium iodide.
6. Explain the formation of approximately the same amount of butylene (20%) and tert-butyl alcohol (80%) during the hydrolysis of (CH 3) 3 C-X (X = Cl, Br, I).
7. Write down all the products that can be expected in the reactions below. Suggest schemes of the mechanisms of their formation:
7.1. 1-chloro-1-methylcyclohexane + ethanol
7.2. 1-chloro-1-methylcyclohexane + sodium ethoxide (in ethanol)
8. Carry out (in two stages) the following transformations:
8.1. butene-2 methyl- second- butyl ether,
8.2. 2-methylbutene-2 2-methoxy-2-methylbutane,
8.3. styrene 1-phenyl-1-cyanoethane.
9. Based on the corresponding alkene, carry out (in two stages) the synthesis:
9.1. 1-phenyl-1-aminoethane,
9.2. (CH 3 CH 2) 2 CHSH.
10. Using the reaction of 1,4-electrophilic addition to conjugated dienes and the reaction of nucleophilic substitution, carry out (in two stages) the following transformations:
10.1. 1,3-butadiene CH 3 CH \u003d CHCH 2 C N,
10.2. 1,3-butadiene N CCH 2 CH=CHCH 2 C N
11. Give the most preferred methods of synthesis:
11.1. n- dibutyl ether,
11.2. ethoxybenzene (phenetol),
11.3. ethyl- tert-butyl ether (industrial method).
11.4. methylbenzyl ether.
12. Explain why the Williamson synthesis cannot be used to obtain diphenyl ether. How is this ether received?
13. Unlike alkanes, ethers dissolve in cold concentrated sulfuric acid. This reaction is a simple chemical test to detect the difference between these classes of compounds. What chemistry is the basis of this difference (write equations).
14. Write an equation for each of the reactions below. Note the cases where the reactions do not go.
14.1. di- n-butyl ether + boiling aqueous NaOH
14.2. methyl- n-propyl ether + excess hot HBr
14.3. di- n-propyl ether + Na
14.5. diethyl ether + cold concentrated H 2 SO 4
14.6. ethylphenyl ether + BBr 3 (1. heating, 2. H 2 O).
15. On heating with excess HBr, the cyclic ether gives 1,4-dibromobutane. Write the structure of the ether and the reaction equation.
16. Get 1,2-butanediol from n-butyl alcohol. Write conditions and schemes of reaction mechanisms.
17. Write the reaction equations for ethylene oxide with:
17.1. 1 HBr equivalent,
17.2. with an excess of HBr,
17.3. phenol and further with dilute acid.
18. 2-Phenylethanol, which smells like rose oil, is used in perfumery. Write how this substance can be synthesized starting from bromobenzene.
19. When 1,1-dimethyloxirane is dissolved in an excess of methanol and the reaction mixture is treated with a small amount of acid, 2-methoxy-2-methylpropanol-1 is formed. Provide a diagram of the mechanism that explains this result. Explain why 1-methoxy-2-methylpropanol-2 is not obtained in this reaction.
20. The first industrial method for the synthesis of ethylene oxide was based on the treatment of ethylene with hypochlorous acid and the subsequent reaction of the product with a dilute base. Write the equations for these reactions and describe their mechanism diagrams.
21. Write the conditions of the scheme of receiving mechanisms:
21.1. 2-methoxyethanol from ethylene oxide and methyl alcohol,
21.2. diethylene glycol from ethylene oxide and ethylene glycol.
22. What chemical tests should be used to distinguish the compounds in each of the following pairs. Indicate what can be visually observed during each reaction-test:
22.1. di- n- propyl ether and n-hexane
22.2. ethyl phenyl ether and allyl phenyl ether,
22.3. 2-butanol and methyl- n- propyl ether,
22.4. phenol and anisole,
22.5. phenol and 1-hexanol.
23. What is the structure of the compound C 4 H 10 O 3 if, when treated with an excess of HBr, the only organic compound, 1,2-dibromoethane, was obtained.
24. Write the structure of the distillate compound obtained by slowly heating a mixture of ethylene glycol with dilute sulfuric acid while simultaneously distilling off the reaction product. Give a series of equations that describe the mechanism of this reaction.
25. Compare the reactions of cyclohexanol and phenol with:
25.1. hbr,
25.2. H 2 SO 4 (heating),
25.3. PCl 3
Formula R-OH, OH functional group attached to an atom
carbon in sp3 hybridization
where R is an alkyl or substituted alkyl group.
Classification by the number of OH groups
monatomic
methanol
Diatomic
ethylene glycol
Triatomic
Glycerol
1Classification by type of carbon atom,
to which an OH group is attached
Primary
Secondary
Tertiary
ethanol
Iso-Propanol
Tert-Butanol
2
Classification according to the structure of the hydrocarbon radical: saturated, unsaturated and aromatic alcohols.
CH3H3C
CH2 C
Saturated
2-Methyl-2-butanol
Oh
CH3
CH3
H2C
CH
C
Oh
CH3
CH3
C
Oh
unsaturated
2-Methyl-2-buten-2-ol
Aromatic
2-Phenyl-2-propanol
CH3
3Nomenclature
According to the IUPAC nomenclature, saturated alcohols are called
alkanols. The name contains the suffix "OL".
5
H3C
Br
4
WITH
3
CH
CH3 CH2
2
CH
1
CH3
5
CH3
4
CH
HE
3
CH
2
CH
1
CH3
HE
CH3
4-Bromo-4-methyl-3-ethyl-2-pentanol
3-Penten-2-ol
According to the radical-functional nomenclature
the name of alcohols is made up of the name of the radical and the word
alcohol.
CH3OH
Methyl
alcohol
CH3CHCH3
HE
isopropyl
alcohol
CH2OH
benzyl
alcohol
HE
Cyclohexyl
alcohol
4How to get
Hydration of alkenes
H2C
CH CH3
+HO
H+
2
H3C
CH
CH3
Oh
AdE mechanism,
pr. Markovnikova
rearrangement possible
Hydrolysis of haloalkanes
Br
H3C
CH CH3
NaOH, H2O, T
-NaBr
Oh
H3C
CH CH3
Mechanism SN1, SN2
If SN1, possible
rearrangement.
Competing reaction:
splitting off (E1,E2)
5Oxymercuration-Demercuration (AdE)
Region-specific production of alcohols according to the rule
Markovnikov. Conjugated connection. Missing
rearrangements
1) Hg(OAc)2; THF-H2O; 20OC
CH3
2) NaBH4; H2O
H3C C CH CH3
H3C C CH CH2
CH3
+
H3COH
3,3-dimethyl-2-butanol
(97%)
CH3
1) Hg(OAc)2; THF-H2O; 20OC
2) NaBH4; H2O
H3C CH2 CH CH2
H3C CH2 CH CH3
3
1-hexene
3
Oh
2-hexanol
(99,5%)
CH3
H3C C CH2 CH2 OH
CH3
3,3-dimethyl-1-butanol
+
H3C CH2 CH2 CH2 OH
3
1-hexanol
(0,5%)
6reaction mechanism
R HC CH2 +
+
HgOAc
RHC
H2 O
+
CH2
Oh
+
R CH CH2 Hg OAc + H
hg
OAC
cyclical
mercury ion
NaBH4
R CH CH2 HgH
Oh
R CH CH3 + Hg
Oh
hydroxyalkylmercurohydride
Conjugated connection. The role of the external nucleophile is performed by
the solvent is water.
7Synthesis with the Grignard reagent
Reaction with aldehydes and ketones. AdN
(H)R1
+
WITH
(H) R2
O
R3
(abs. ef.) (H) R1
+
MgBr
(H) R2
H2O, HCl
WITH
O
R3
MgBr
-MgBrCl
(H)R1
(H) R2
WITH
Oh
R3
Formaldehyde→primary alcohol
Aldehydes→secondary alcohol
Ketones→tertiary alcohol
abs.
H
H2O, HCl
ether
CH3CH2CH2OMgBr
CO+CH3CH2MgBr
CH3CH2CH2OH
H
propoxymagnesium1-propanol
bromide
abs.
CH3
CH3
H3C
ether
H2O, HCl
CO+CH3CH2MgBr
H3C H2C HC OMgBr
H3C H2C HC OH MgClBr
H
2-butoxymagnesium2-butanol
bromide
abs.
CH3
CH3
H3C
H2O, HCl
ether
CO+CH3CH2MgBr
H3C H2C C OMgBr
H3C H2C C OH
H3C
CH3
CH3
2-methyl-2-butoxymagnesium2-methyl-2-butanol
bromide
8Reaction with ethylene oxide
CH2
O
+
CH2
(abs. ef.)
CH2
CH2
R1
R1
H2C CH2
O
+
MgBr
H2O, HCl
O
-MgBrCl
R1
CH2CH2OH
MgBr
An alcohol molecule is two carbon atoms larger
than in the Mg-organic compound.
(abs. ef.)
MgBr
H
C
H
C
+ 3 2
ethylmagnesium bromide
H2O, HCl
H3C CH2 CH2 CH2 O MgBr
-MgBrCl
butoxymagnesium bromide
H3C CH2 CH2 CH2 OH
1-butanol
9Reaction with esters
O
C6H5
+
WITH
C6H5
OS2H5
ethyl benzoate
(abs. ef.)
C6H5
+
(abs. ef.)
MgBr
C6H5
- С2Н5ОMgBr
O
+
WITH
C6H5
+
MgBr
C6H5
O
MgBr
WITH
C6H5
C6H5
HE
H2O, NH4Cl
-MgBrCl,
-NH4OH
C6H5
WITH
C6H5
C6H5
Triphenylmethanol
Fermentation of sugars
enzyme
C6H12O6
2 C2H5OH + 2 CO2
10Hydroboration-oxidation of alkenes
1) BH3
2) H2O2,NaOH-H2O
Oh
CH3CH2CHCH2OH
CH3CH2C CH2
+
CH3CH2C CH3
CH3 (99%)
CH3
(1%) CH3
reaction mechanism
CH3 CH CH2
CH
CH3
H B H
H
CH2
CH3 CH2 CH2
CH3 CH CH2
BH3
H B H
H
H
CH3 CH CH2
H
CH3 CH2 CH2 B
3
2 CH3 CH CH2
CH3 CH CH2
B
H
H
H
H
H
CH3 CH2 CH2 B
BH2
H2O2, OH
B
CH2 CH2 CH3
CH2 CH2 CH3
Tripropylboron
3 CH3 CH2 CH2 OH
+ B(OH)3
11reaction mechanism
q-charges on atoms
q = - 0.2260
q = - 0.1619
CH3
CH3
CH CH2
H BH2
Electronegativity
hydrogen 2.1 > boron 1.9
Steric factor
CH3
CH3
CH2
H
BH2
CH3
CH
H
CH2
H
PS1
BH2
CH
+
CH2
H2B
Rboron > Rhydrogen
+
CH
+
CH
Localization of the positive
charge on the secondary atom
carbon (PS1) is more profitable,
than on the primary (PS2)
CH2
BH2
H
CH3
PS2
CH
CH2
H
BH2
12Recovery of carbonyl compounds
Aldehyde→primary alcohol
Ketone→secondary alcohol
Recovery of aldehydes and ketones
O
H2, Ni
CH3 CH CH C
CH3 CH2 CH2 OH + C4H10
2
H
Selective reduction of the carbonyl group
H
O
CH3 CH CH C
+
+
H
Al
H
Li
H
O
CH3 CH CH CH2 O
H
Al
O
CH2 CH CH CH3
O
CH2 CH CH CH3
+
Li
10% H2SO4
CH2 CH CH CH3
3+
4 CH3 CH CH CH2 OH + Al + Li
+
13Recovery of aldehydes and ketones
O
CH2 CH CH2CH2C
H
NaBH4
C2H5OH
CH2CH CH2CH2CH2OH
4-n ten -1-o l
4-p en ten al
O
(85%)
1) LiAlH4, ýô ðr, 0-10 Î Ñ
Oh
2) H2O, H+, 0OC
(94%)
2-cyclohexene -1-o l
2-cyclohexane -1-o n
LiAlH4 reduction mechanism
R1
CO
+
+ LiAlH4
R2
R1
+
H C O Al Li
R2
4
R1
+
H C O AlH3 Li
R2
H2O
R1
4 H COH
R2
+ Al(OH)3 + LiOH
14O
1) NaBH4, C2H5OH
2) H2O, H+
Oh
Oh
+
H
cyclohex-2-en-1-one
(59%)
DIBAL-N,
benzene, 10 OS
O
tricyclodec-4-en-3-one
H
(41%)
(90%)
Oh
tricyclodec-4-en-3-ol
CH3
CH3
CH3 CH CH2
CH2 CH CH3
AlH
DIBAL-N
15Recovery of carboxylic acids
O
CH3
CH2
C
1) LiAlH4
2) 10% H2SO4
14
palmitic
acid
CH3
CH2
14
CH2
Oh
Oh
1-Hexadecanol
Recovery of esters to primary alcohols. Bouveau-Blanc reaction
Na+C2H5OH
O
CH3 CH2
C
14
O
CH3
1) LiAlH4
2) 10% H2SO4
CH3 CH2
CH2OH
14
+CH3OH
Methyl palmate
Recovery of carbon monoxide. industrial method
CO + 2H2
Cu-ZnO-Cr2O3 , T
CH3OH
16Physical properties
Comparison of the physical properties of alcohols and hydrocarbons
Alcohol
Hydrocarbon
Molecular Tm. OS
mass
Tbp. OS
Solubility in
100 ml water, ml
CH3OH
CH3 CH3
32
30
-98,0
-172,0
65,0
-89,0
Unlimited
4,7
CH3CH2OH
CH3CH2CH3
45
44
-117,3
-189,9
78,5
-42,2
Unlimited
6,5
CH3CH2CH2OH
CH3CH2CH2CH3
60
58
-127,0
-135,0
97,2
-0,6
Unlimited
15,0
17The structure of the alcohol molecule
H
Attack
nucleophile
Nucleophilicity
Basicity
+
C
....
O
Acidity
H+
H
H
OH group substitution
for nucleophile (SN)
Cleavage of OH group(E)
ECO=82 kcal/mol
EOH=111 kcal/mol
COH=107-109O
18Hydrogen bonds
E=3-6 kcal/mol
19Chemical properties
1. Acidity of alcohols
CH2
CH3
CH2
Oh
CH2
CH3
Oh
CH3
+ Na
O
CH2
CH3
+
ONa
H
+
+ 1/2 H2
Na ethoxide
Na ethylate
CH2
CH3
ONa
+
H2O
Stronger
acid than
ethanol
CH2
CH3
OH+NaOH
less strong
acid than
water
20Acidity of alcohols in aqueous solutions
ROH
R
O
+
H
+
pKa
tert-butanol
18,0
ethanol
15,9
methanol
15,5
Water
15,7
FCH2CH2OH
13,9
CF3CH2OH
12,4
(CF3)3COH
5,0
Ka
pKa=-lgKa
+I-effect
alkyl groups
-M-effect F
212. Basicity of alcohols
Basicity of alcohols - the ability to attach a proton
....
H
R O H + H
..
+
+
R O H
...
H
Nu
+
R+
O H
Water is good
leaving group
The nucleophilicity of alcohols is the ability to form
bonds with other atoms through lone pairs of electrons.
....
....
R O Na
+
Strong
nucleophile
R O H + H
H
+
Weak
nucleophile
..
+
R O H
Alkylhydroxonium ion
Strong
electrophile
H
+
R+
...
O H
Water is good
leaving group
22Base alcohols.
They form salts with Bronsted and Lewis acids
H
CH3OH + HBr
+
CH3 O
HBr
Methylhydroxonium
bromide
H
CH3 OH + AlCl3
+
CH3 O
AlCl3
Influence of the structure of alcohols on acid-base properties
CH3
CH3OH
CH3CH2OH
increase in basicity
increase in acidity
CH3CHOH
CH3
CH3 C
Oh
CH3
+I-effect of alkyl groups
233. Alcohols-nucleophilic agents
Getting ethers
CH3CH2OH + HOCH2CH3
H
Mechanism of the SN2ac reaction
H
CH3CH2O:
CH3
C
H
Nucleophile
+
CH3CH2O
H
H
H
H
+
CH3CH2
O
CH2CH3 + H2O
diethyl ether
Competing reaction E
The reaction is reversible
CH3
+
OH2
H
substrate
CH3
C
+
+
CH3CH2OH2
+
CH3CH2OH +
primary alcohols.
Intermolecular dehydration
H2O
"
CH3CH2O
C
"
OH2
-H2O
H
H
H
transition state
CH3CH2 O
CH2CH3
+
+
H3O
24Obtaining ethers. Synthesis of A. Williamson.
Symmetric and non-symmetric ethers
SN2
+
С2Н5О Na + CH3I
C2H5OCH3+
Methylethyl
Strong
ether
nucleophile
NaI
Competing reactions
E2
H
CH3O
+
Na
strong
base
Strong
nucleophile
CH2
CH I
CH3
CH3 CH = CH2 + CH3OH
SN2
CH3CH
OCH3 + NaI
CH3
No competing reaction
CH3
CH O
+
CH Na
3
I
+
CH3
SN2
CH3CH
OCH3 + NaI
CH3
Strong
nucleophile
strong
base
25Obtaining ethers. Reaction of alcohols with alkenes.
CH3
H3C
C
CH3
+
+
CH2HO
H
-H2O
CH3
H3C
C
O
CH3
CH3
High octane additive
SN1ac reaction mechanism
H3C
C
CH3
H3C
C
....
CH3
CH3
+H
CH2
+
H3C
C
+
H
CH3
+
O
CH3
CH3
+
HO
CH3
Nucleophile
CH3
H3C
C
O
CH3
+H
+
CH3
26Obtaining esters. esterification reaction.
CH3 C
O
X
X=
+
H
Cl
O
OC
CH3
Oh
..
O
..
H
O
+
CH3
CH3 C
Methanol nucleophile
+
HX
O
CH3
Methyl acetate
Carboxylic acid chlorides
Anhydrides of carboxylic acids
carboxylic acids
Optically active alcohols react without breaking bonds at the chiral atom,
hence the product will have the configuration of the original alcohol
CH3 C
O
+
H
Oh
..
O
..
CH3
*
CH
H
O
+
CH2 CH3
(S)-2-Butanol
Nucleophile
CH3 + H2O
CH3 C
O
*
CH
CH2 CH3
(S)-2-Butyl acetate
27Esters of mineral acids
O
2CH3OH
+
H2SO4
H3C O S O CH3
+
2H2O
O
O
2CH3OH
+
ClSO2OH
H3C O S O CH3
Õëî ðñóëüô î í î âàÿ
êèñëî òà
O
+
H2O
+ HCl
Äèì åòèëñóëüô àòàëêèëèðóþ ù èé àãåí ò
O
CH3OH
+
ClSO2OH
Õëî ðñóëüô î í î âàÿ
êèñëî òà
CH3OH
+ HNO3
H3C O S OH
+
H2O
+ HCl
O
Methylsulphate
H3C O NO2 + H2O
Ì åòèëí èòðàòâçðû â÷àòî å
âåù åñòâî
284. Nucleophilic substitution of the OH group
The conversion of alcohols to halogen derivatives
Reagents:
Hydrogen halides (HCl, HBr, Na(K)Br+H2SO4, Na(K)I+H2SO4)
Phosphorus chlorides, bromides (PCl3 PBr3)
Thionyl chloride (SOCl2)
A mixture of phosphorus and iodine
Phosphorus oxychloride (POCl3)
CH3CH2CH2CH2OH
CH3CH2CH CH3
Oh
CH3
CH3 COH
2h
NaBr+H2SO4, 60OC
HCl, H2O, 20OC
CH3
CH3
Oh
48% HBr+H2SO4, 120OC
10 min
HCl (gas), 0 OC
ether
CH3CH2CH2CH2 Br + H2O
(95%)
CH3CH2CHCH3 + NaHSO4+ H2O
(60%)Br
CH3
CH3 C Cl + H2O
CH3
CH3
(90%) + H2O
Cl
29Substitution of an OH group by a halogen under the action of hydrogen halides
C
Nu
ROH
+
HX
SNac
Oh
RX
HI > HBr > HCl
+ H2O
X= Cl, Br, I
reactivity decreases.
SN2ac mechanism. primary alcohols.
H
CH2. .
+
+ H
Oh
CH3
.
Cl
.
CH3
CH2
Cl
+ H2O
H
H
C. + slowly
+H
Cl
C
O
.
Oh
CH3
.
H
H H H
H
Attack from the rear
In the case of optical
active alcohol inversion configuration
30SN1ac mechanism. Secondary, tertiary alcohols.
H
C2H5
*CHOH
+
Optically
CH3
active
alcohol
slowly
-H2O
H
+
+
C2H5 *CHOH
CH3
H
C
Cl
+
C2H5*CH
C2H5
Cl
CH3
Racemate
CH3
carbocation,
nucleophile attack
on both sides
regrouping
CH3
CH3CH CH CH3
H
+
CH3C
H
1,2-H-shift
CH CH3
+
CH3CH CH CH3
H
Oh
CH3
CH3
+
O
slowly
-H2O
LUMO carbocation
H
CH3
CH3
+
+
H3C C CH2 CH3 Br
more sustainable
tertiary carbocation
H3C
C
CH2CH3
Br
31Reactivity of alcohols with respect to hydrogen halides
Benzyl, allyl > tertiary > secondary > primary > methanol
SN1
The stability of the carbocation increases, the reactive
ability increases
SN2
Spatial
obstacles to attack
decreasing from the rear
reactionary
ability
increases
32Reaction with phosphorus halides PCl5, PCl3, PBr3, PI3,
thionyl chloride SOCl2.
CH3
Br
CH
PBr3
CH3
Oh
CH
CH3
CH
CH3
CH3
PCl5
+
P(OH)3
CH3
Cl
CH
CH
CH
CH3
SOCl2
CH3
CH3
3-Methyl-2-butanol
CH3
Cl
CH
CH3
+ POCl3 + HCl
CH
+
SO2
+ HCl
CH3
33Reaction of optically active alcohols with thionyl chloride.
benzene, ether
H5C2
H
H3C
Oh
R-2-Butanol
H5C2
Cl
H
H3C
R-2-Chlorobutane
SOCl2
Preservation
configuration
H5C2
pyridine
Cl
H
CH3
Appeal
configuration
S-2-Chlorobutane
34The mechanism of the reaction of alcohols with thionyl chloride.
Reaction without reason. SNi mechanism.
(i-internal)
CH2
CH3
CH2
CH3
+
CH
-HCl
Thionyl chloride
Oh
H
+
CH2 O
CH3
O
Cl
S
CH3
Cl
C
CH
O
2-Pentylchlorosulfite
2-Pentanol
H3C
CH3
SOCl2
CH3
H
S
O
Close ion pair.
Attack from the front.
-SO2
C
Cl
CH2
CH3
Preservation
configuration
35.
+
O
O
R O S
SN2
Cl 1,4-dioxane
O
+
O R + Cl
+SO2
appeal
configuration
Cl
+
R O
+
rear attack
O
SN2
RCl
+
O
O
appeal
configuration
See slide notes
36reaction in the presence of a base. SN2 mechanism.
C2H5
H
C
Oh
CH3
+
H5C2
N
SOCl2
HN
Cl
Thionyl chloride
R-2-Butanol
+
+
H
CO
Cl
HN
S
CH3
+
O
Attack from the rear
O
C2H5
Cl
C
H
+
+
+
S
HN
O
Cl
Appeal
hydrochloric acid
configuration
pyridine
CH3
37Reaction of alcohols with PCl3 and PBr3.
CH3
PCl3
CH3
3
CH3
CH3
H
CH
C*
CH3
CH3
*
C
CH
Cl
H
+
CH3
CH
CH3
Oh
PBr3
H
C*
CH3
POH
2
CH3
CH3
3
C*
CH
Br
+
P(OH)3
H
CH3
Configuration inversion
38The mechanism of the reaction of alcohols with PBr3.
H3C
3
H3C
C
+
Oh
H
C3H7-i
PBr3
C
-3HBr
R-3-Methyl-2-butanol
CH3
Br
C
H
O P
C3H7-i
H
C3H7-i
H3C
+
O P
H Br 3 Br
SN2
3
tri(1,3-dimethylpropyl)phosphite
H
C3H7-i
3
CH3
C
transition state
P
Consistently
three times Branion attack from the rear
CH3
H
C3H7-i
CO
3Br
2
C
H
C3H7-i
+
P(OH)3
Configuration inversion
The BrΘ anion is a more active nucleophile than the ClΘ anion
395. Elimination of the OH group
Obtaining alkenes. intramolecular dehydration
H2SO4, T
CH3
CH
CH2 CH3
E1ac
Oh
2-Butanol
HE
CH3
C
H2SO4, T
CH2
CH3
E1ac
CH3
2-Methyl-2-butanol
(tert-Pentyl alcohol)
CH3 CH = CH2 CH3 + H2O
trans-2-butene
(main product)
CH3
C=CH
CH3 + H2O
CH3
2-Methyl-2-butene
Zaitsev's rule
40primary alcohols.
CH2
CH3
Oh
CH2
96% H2SO4, 180OC
H3C
HC CH2
-H2O
E2ac reaction mechanism
..
..
1) CH3 CH2 OH + H
+
CH2 CH2
H
..H
..OH
+
H2C
CH2 + H2O + H2SO4
OSO2OH
Synchronized: water separation
and proton
O
2) RCH2CH2OH + H2SO4
O
RCH2CH2
HSO4-
O SOH + H2O
O
RCH CH2 + H2SO4 + HSO4R CH CH2 O S OH
170 - 190OC
O
H
41Secondary, tertiary alcohols.
E1ac mechanism. Possible rearrangement
CH3
C
CH3
CH3
CH3
CH3
H2SO4, 80 OS
CH
Oh
3-Methyl-2-butanol
CH3
CH3
CH
CH
CH3
+OH
C
+
CH
CH3
More sustainable
tertiary carbocation CH3
C
CH3
+
1,2-CH3-shift
CH3
H
2
CH3
CH3
CH3
CH3
-H+
C
CH3
CH
CH3
more stable, more
alkylated alkene
Zaitsev's rule
The main product of the cleavage reaction from haloalkanes with
two non-equivalent C-atoms is the most
stable (most alkylated, thermodynamically
stable) alkene.
42Reaction direction of catalytic dehydration of alcohols
H3C
CH2 CH2
5
CH CH3
Oh
300-400OC
ThO2
Al2O3
H3C
CH2 CH
5
according to Zaitsev
CH CH3
H3C
CH2 CH2 CH
5
CH2
according to Hoffmann
43Alcohol oxidation
Primary alcohols are oxidized to aldehydes, then to acids
H3C
OH[O]
CH2
O
CH3 C
[o]
H
Acetaldehyde
ethanol
O
CH3 C
Oh
Acetic acid
Secondary alcohols are oxidized to ketones
H3C
O
[o]
CH
Oh
H3C
iso-propanol
CH3 C
CH3
Acetone
Tertiary alcohols are oxidized with the destruction of the skeleton
44Examples of oxidation of primary alcohols to aldehydes
Oxidizing agent: complex of chromium (IV) oxide with pyridine (Sarett-Collins reagent)
.
O
N
+
CH2
Oh
O
4-Nitrophenylmethanol
4-nitrobenzyl alcohol
CH2Cl2 ;
O
O
CrO3 2C5H5N
N
25OC
+
(97%)
C
O
H
4-Nitrobenzaldehyde
Oxidizing agent: Saretta's reagent (pyridinium chlorochromate,
very soluble in organic solvents)
H3C
CH2 C
4
C
CH2OH
3-Octin-1-ol
.
.
CrO3 C5H5N HCl
CH2Cl2 ; 25
OC
O
H3C
CH2 C
C
4
2-Octinal
(84%)
C
H
Oxidizing agent: manganese (IV) oxide.
O
CH2OH
CH3 CH2
C
H
C
H
Z-2-Buten-1-ol
MnO2 20OC
CH2Cl2 or C6H14
C
CH3 CH2
C
H
C
H
H
Z-2-Butenal
45Examples of oxidation of primary alcohols to carboxylic acids
Jones reagent (solution of CrO3 in aqueous H2SO4).
O
CH2OH
C
CrO3-H2O-H2SO4
acetone, 20 OS
C6H5
(1-Phenylcyclopentyl)methanol
Oh
C6H5
1-Phenylcyclopentanoic acid
Examples of oxidation of secondary alcohols to ketones
Oh
CrO3-H2O-H2SO4
O
acetone, 20 OS
Cyclooctanol
Cyclooctanone
46Oxidation of tertiary alcohols with destruction of the skeleton in an acidic medium
CH3
CH3 C
Oh
CH3
H
+
-H2O
CH3 C
CH2
[o]
CH3 C CH3
+ CO2 + H2O
O
CH3
Catalytic dehydrogenation of alcohols
O
Cu or Ag, 600 OS
H3C
Oh
Industrial
process.
Reaction Example
α-elimination.
HC
-H2
methanol
H
Formaldehyde
Oh
Cu, 600OC
O
-H2
Cyclohexanol
Cyclohexanone
47Alkylation of SEAr arenes
..
CH.OH
. +BF
3
3
+
H3C
H
O
+
CH3 (BF3OH)
bf3
CH3
+
CH3 (BF3OH)
+
SEAr
+ H2O + BF3
Representatives of the class of alcohols:
Methanol - poison, solvent, reagent in syntheses
Ethanol - a poison in large quantities, a solvent, a reagent in syntheses
Iso-Propanol - solvent, reagent in syntheses
48The main directions of chemical transformations of alcohols
Dehydration
SEAr
Nucleophilic
substitution
Alcohol
Dehydrogenation
Oxidation
Alkenes, ethers
Alkylaromatic
connections
halogen derivatives,
ethers,
esters,
Aldehydes, ketones
Aldehydes, ketones,
carboxylic acids
49Diols (dihydric alcohols)
CH2
HO
Oh
CH2 CH2
OH OH
H3C CH CH2
OH OH
Methanediol - does not exist in free form
1,2-Ethandiol (ethylene glycol)
1,2-Proandiol (propylene glycol)
Oh
trans-1,2-Cyclohexanediol
Oh
50How to get
Hydroxylation of alkenes with Mylas reagent, hydrogen peroxide,
according to the Wagner reaction, the Krieg reaction
Oh
H2O2, OsO4 , 0OC
H
Oh
(Mylas reagent)
H
cis-1,2-Cyclohexanediol
H2O2, CH3COOH, H2SO4
Oh
KMnO4, H2O, 20OC, pH=7
H
(Wagner reaction)
Oh
1) OsO4, 25OC
H
2) NaHSO3/H2O
trans-1,2-Cyclohexanediol
(Krige reaction)
51Preparation of ethylene glycol from ethylene.
Ca(OH)2
Cl2, H2O
CH2 CH2
H2C
CH2 CH2
H2O, H+
CH2
CH2 CH2
ClOH
O
OH OH
Ethylene chlorohydrin Ethylene oxide
H2O, NaOH
Obtaining glycerin from propylene.
NaOH, H2O
Cl2, 400
CH2 CH CH3
OC
CH2 CH CH2
Cl
H2O2, H2O
CH2 CH CH2
Oh
Cl2, H2O
NaOH, H2O
CH2 CH CH2
OH OH OH
CH2 CH CH2
ClOHCl
52Classical reductive dimerization of ketones
(Pinacon restoration).
CH3 C
CH3
1) Mg, benzene
2) H2O
CH3
CH3 C
CH3
CCH3
OH OH
(43-50%).
Pinacon
2,3-Dimethyl-2,3-butanediol
O
Flaws:
low exit,
enter into a reaction
only ketones.
Modern reductive dimerization of ketones
in the presence of TiCl4 in THF (I. Kori).
O Zn, TiCl4 THF, C6H5
25 OS, 2 hours
C
H3C
CH3
O
n-C7H15 C
H
Mg(Hg), TiCl4, THF,
0 OS, 13 h
C6H5
C
C
OH OH
(91%)
CH3
Benefits: high
output, can react
enter not only ketones,
but also aromatic
aliphatic aldehydes
n-C7H15 CH CH n-C7H15 (80%)
OH OH
8,9-Hexadecanol
53Preparation of 1,3-diols by reduction of aldols.
O
R1 CH CH2 C
Oh
NaBH4, DME
R2
R1 CH CH2 CH R2
Oh
Oh
DME-dimethoxyethane
1,3-Diol
Chemical properties
For polyhydric alcohols, the same reactions are characteristic as for
monohydric alcohols.
Dehydration of 1,2-diols.
Dehydration of 1,2-diols to 1,3-dienes.
CH3
CH3
C
CH3
C
OH OH
CH3
Al2O3, 450-470OC
H2C
C
C
CH2
(70-85%)
CH3 CH3
54Dehydration of 1,2-diols with rearrangement
(pinacoline rearrangement of 1,2-diols).
CH3 CH3
CH3 C
C
CH3
H2SO4, tOC
CH3
CH3 C
OH OH
C
CH3 + H2O
R. Fittig's reaction.
OCH3
Pinacolin
Pinacon
35% H2SO4, tOC
CH3 CH CH CH3
CH3 C
OH OH
CH2 CH3
(81%)
O
butanone
The mechanism of the pinacol rearrangement.
R1
RC
R1
C
H
+
R
R
OH OH
R1
R1
C
C
R
R
R
C
C
+
+OH
R1
R
H
C
+
C
..OH
OH OH2
R1
R1
-H2O
R
R1
R1
+
R
C
C
O
R1
R
55Dehydration with the formation of cyclic ethers.
Intermolecular dehydration
Oh
H2C
H2C
HO
+
Oh
HO
O
CH2 H2SO4, conc., 140 OC H2C
CH2
H2C
CH2
CH2
(50-55%)
O
1,4-Dioxane
Intramolecular cyclodehydration
with the formation of cyclic ethers.
HO
CH2
5
Oh
1,5-Pentanediol
57% H2SO4
- H2O
O
(100%)
tetrahydropyran
56
Classification and nomenclature of ethers
EthersClassification and nomenclature of ethers
By structure
hydrocarbon
radicals
H3C
Nomenclature
IUPAC
Symmetric,
dialkyl
diethyl
ether
2-Ethoxyethane
asymmetrical,
dialkyl
Methylethyl
ether
Methoxyethane
asymmetrical,
alkylaryl
Methylphenyl ether
Methoxybenzene
Symmetric,
diaryl
Diphenyl
ether
Phenoxybenzene
Tetrahydrofuran
Tetrahydrofuran
O
Cyclical,
alkyl
Furan
O
Cyclical,
aromatic
Oxacyclopentadiene
O
CH2
CH2
H3C
Trivial
nomenclature
CH3
CH2
CH3
O
O
CH3
O
57How to get
Intermolecular dehydration of alcohols.
Symmetrical dialkyl ethers.
CH3CH2OH + HOCH2CH3
H
+
CH3CH2
O
CH2CH3 + H2O
diethyl ether
Williamson reaction.
Symmetrical and unsymmetrical dialkyl and alkylaryl ethers
SN2
+
С2Н5О Na + CH3I
C2H5OCH3+
Methylethyl
Strong
ether
nucleophile
NaI
NO2
O2N
Br
NO2
+
H3C
OK
+
DMF
-KBr
O2N
OCH3
1-Methoxy-2,4-dinitrobenzene
58Getting crown ethers
ONa
ONa
+
O
CH2
CH2
CH2
CH2
Cl
Cl
Cl
CH2
O
+
Cl
CH2
CH2
O
Disodium
CH2
catechol salt
(Disodium 1,2di-(2-chloroethyl) ether
benzene diolate)
Oh
C4H9OH-H2O
ONa
100
ONa
OC
+
O
O
Dibenzo-18-crown-6
O
KOH, H2O-THF
O
Oh
(45%)
first crown air,
K. Pederson (1967)
O
O
O
O
Cl
O
O
O
O
O
O
(Ä.Êðàì)
O
Cl
18-crown-6
(40-60%)
Reaction of alcohols with alkenes.
CH3
H3C
C
CH3
+
+
CH2HO
H
CH3
-H2O
H3C
C
O
CH3
CH3
Methyl tert-butyl ether (MTBE).
High octane additive
59physical properties.
T pl., OS
T boil., OS
CH3OCH3
-138,5
-23,2
CH3CH2OH
-117,3
64,7
CH3CH2OCH2CH3
-116,3
34,6
CH3CH2CH2CH2OH
-89,5
117,7
The structure of the ester molecule
.. ..
H
Weak bases
Weak nucleophiles
0.142 nm
H
O
C
111O
C
H
1) Reactions at the oxygen atom
2) Reactions at the a-carbon atom
3) C-O bond cleavage reactions
H
H
H
60Chemical properties
Reactions at the oxygen atom
R
R1
..
Oh.. + HX
R
X=Cl, HSO4 R1
..
Oh
.
protonation under the action
weak acids. Education
hydrogen bonds.
HX
Formation of charge transfer complexes (CTC) with strong acids.
R
..
Oh.. + HX
R1
(C2H5)2O
X=Br, I
+BF3
+
R
Oh
.
H X
Bronsted acids
R1
Dialkyloxone cation
+
(C2H5)2O
bf3
Lewis acids
Etherate trifluoride
boron. Complex with transfer
charge (bullpen)
61Formation of trialkyloxonium salts.
C2H5
..
.. + CH
O
C2H5
R
R
2
5
F
C2H5
+BF3
..
O
+
C2H5 BF4
C2H5
Trifluoroborate
triethyloxonium
.O+.
R
+ .. Nu
Nu
bf4
bf4
C2H5
R
+
..
O
+
R
..
..
R
+
C2H5 BF4
C2H5
ROH
Strong
alkylating agent.
Reacts with
weak nucleophiles.
O
C2H5
ROC2H5
+
C2H5
..
.. +
O
bf3
Trifluoroborate
triethyloxonium
62Reactions at the a-carbon atom
Alkoxy radical - more
stable than alkyl,
due to the delocalization of the unpaired electron.
SR mechanism.
R
..
O
..
H
H
C
R1
+
H
R
R
-HR
..
O
..C.
R1
Radical halogenation
CH3
CH2
O
CH2
CH3+
Cl2
h , -20
Cl
OC
-HCl
CH
CH3
O
CH2
CH3
1-Chloro-1-ethoxyethane
63Autooxidation
R
.. H
O
.. C
R1
H
R
.. H
OC.
..
R
.. H
O
.. C
R1
R1
O O
+R
-HR
+. O
Oh
+R
.. H
OC
..
H
R
.. H
OC.
..
R
R1
R1
.. H
OC
..
R1
O O
R
.. H
OC
..
R1
OOH
+R
.. H
OC
.
..
R1
Hydroperoxides easily detonate when heated and on impact.
64C-O bond cleavage reactions.
Reaction conditions:
1) Conc. HBr, HI; 120-150OC
2) BCl3, BI3; -20 OS
X
R
..
O
+
SN2
R
X
+
R1
Oh
R1
H
Protonated - SN1
ether
+
R O R1 X
Unprotonated ether
R1
+
+R
X
R
Oh
R1
X
X
+R
O
R,R1-primary,
secondary alkyl,
phenyl.
SN2 mechanism.
Weak base.
Alcohol Good leaving
group
R-primary,
secondary alkyl,
phenyl.
R1-tertiary
alkyl, allyl,
benzyl.
SN1 mechanism.
alkoxide
anion
Strong foundation.
bad leaving
group.
65....
SN2
+H
C6H5 O C2H5
Ethoxybenzene
Fenetol
..
H3C
Br
H
H
C
..
+
+
C6H5 O
H
CH3
Br
+
O
C
H
H
C2H5
..
O C6H5
H
CH3
Br
H
C
H
H
+
HO
C6H5
SN1
....
CH3
CH3 C
O
CH3
+
H
CH3
..
CH3 C
+
O
CH3
H3CH H
slow -CH3OH
MTBE
CH3
CH3 C
+
CH3
Oh
CH3
+
+ CH3
CH3
CH3 C
+
CH3
+
Br
quickly
CH3
CH3 C
Br
CH3
66Ethers do not react with Na at low temperatures.
At elevated temperatures, ethers (especially higher homologues)
split according to the equation
R
O
R1
+ 2 Na
R ONa
Na alkoxide
+
R1 Na
alkyl sodium
Active alkyl sodium can react
with diethyl ether (P.P. Shorygin)
H5C2
O
C2H5
+HC
5
2
Na
H5C2 ONa
Na ethoxide
+
C2H6
+
C2H4
The use of ethers.
Solvents. Synthesis of various complexes. Antiknock additives
to fuel.
67Cyclic ethers.
CH2
CH2
O
ethylene oxide,
oxirane
CH3 CH
CH2
O
Oxide
propylene, 2methyloxirane
CH2 CH2
CH2 O
1,3-epoxypropane,
oxetane
CH2 CH2
CH2
CH2
O
tetrahydrofuran,
tetramethylene oxide
O
CH2
CH2
CH2
O
CH2
1,4-dioxane
68Osirans (Epoxides).
How to get
Direct oxidation of ethene.
H2C
CH2
+ O2
Ag
H2C CH2
250-300 OC
industrial way
obtaining ethylene oxide
Epoxidation of alkenes (reaction of N.A. Prilezhaev).
R
CH CH2
+
O
R
C
O
ether, benzene,
CH2Cl2
Oh
+
CH2Cl2
O OH 0
Cyclohexene
Cl
CH CH2
+
R
C
O
Oksiran
Peracid
(peracid)
O
R
O
O
OC
(80%)
Oh
O
+
Oh
Cl
m-Chloroperbenzoic-7-Oxabicycloheptane
(Cyclohexene oxide,
naya acid
cyclohexene oxide)
69Dehydrohalogenation of halohydrins.
O
H3C
HC CH2
+
NBr
NBS
O
H2O, DMSO
Oh
H2C
Br
HCCH3
B..
H3C
HC CH2
O
70The structure of the molecule
H
0.150 nm
H
C
H
C
O
61O
H
0.146 nm
Energy
voltage
105 kJ/mol
(25 kcal/mol)
H2C
CH2
..O..
H
Attack
nucleophile
+
71Chemical properties
Interaction with nucleophiles
Reactions with weak nucleophiles. Acid catalysis
+
H2C
H, H2O
CH2
O
CH2 1,2-ethanediol
ethylene glycol
OH OH
H2C
+
H3C HC
CH2
H , CH3OH
H3C HC
O
CH2 O CH3
Oh
1-Methoxy-2-propanol
Methyl cellosolve
SN2 mechanism
CH2+H
RHC
O
+
RHC
..
CH2+HO
H
CH3
+O
H
Attack by nucleophile over
accessible by steric
atomic considerations
R
+
CHCH2O
Oh
CH3 -H+
R
CHCH2O
CH3
Oh
72Mechanism SN1
H3C
H3C
+
CH
C
2
Oh
less stable
primary carbocation
quickly
H3C
C
H3C
CH2
H
O
+
H3C
H3C
slowly
CH2
C
H3C
+
O
H
H
H3C
O
C
+
CH3
H
+
H3C
+
C
CH2
+
.
.
HO
CH3
Oh
more sustainable
tertiary carbocation
CH3
CH3
CH2OH
H3C
O
C
CH2OH
CH3
2-Methyl-2-methoxy-1-propanol
73Reactions with strong nucleophiles. SN2 mechanism.
C2H5ONa
C2H5OH
CH2 CH2 O C2H5
Oh
NaOH, H2O CH CH
2
2
OH OH
H2C
CH2
NH3
O
CH2 CH2
2-Ethoxyethanol
Monoethyl
ethylene glycol ether
1,2-Ethanediol
ethylene glycol
2-Aminoethanol
OH NH2
NH2C2H5
CH2 CH2 NH C2H5
2-N-ethylethanolamine
Oh
CH3MgI
CH2 CH2 CH3 + MgIOH
Oh
74SN2 mechanism
..
..
CH3 CH CH2 + NaO CH3
Na methoxide
O
CH3 CH CH2 O CH3
O Na
CH3OH
+
CH3 CH CH2 O CH3 + CH3ONa
Oh
..
H3C
C CH2 + H2N CH3
H3C
O
CH3
H
+
H3C C CH2 N CH3
O
H
CH3
H3C C CH2 NH CH3
Oh
2-Methyl-1-methylamino-2-propanol
75Polymerization
HO
+
H2C
CH2
HO CH2 CH2 O
O
H2C
H2C
n
CH2
O
CH2
O
HO CH2 CH2 O CH2 CH2 O
HO CH2 CH2 O
H
n+2
Polyethylene glycol,
carbovax
Application
Initial reagents for the synthesis of various compounds.
76Phenols
Oh
Oh
Oh
HO
Oh
Oh
Phenol,
monatomic
phenol
hydroquinone,
diatomic
phenol
fluroglucinol,
triatomic
phenol
77How to get
Hydrolysis of aromatic halogen derivatives
Non-activated substitution-arine mechanism
Cl
ONa
+ NaOH
Oh
HCl
360oC, P
-NaCl
Chlorobenzene
Phenol
Activated substitution, SNAr
Cl
ONa
+ 2 NaOH
NO2
p-Nitrochlorobenzene
Oh
160oC, P
HCl
-NaCl,
-H2O
-NaCl
NO2
NO2
p-Nitrophenol
78Preparation of phenol from isopropylbenzene hydroperoxide
H
O O
H3C CH3
H3C CH CH3
O2
Oh
Hydroperoxide
cumene
Cumol
O
Н2SO4, H2O
+
H3C CH3
Phenol
Acetone
Substitution of the sulfonate group, alkaline melt of sulfonates
SO2ONa
300OC
+
Oh
ONa
NaOH
-Na2SO3
H2SO4, H2O
-NaHSO4
Benzenesulfonate
sodium
79Substitution of a diazo group by a hydroxyl
+
NH2
N2OSO3H
NaNO2 + 2H2SO4
H2O
+ N2 + H2SO4
5oC
Aniline
Oh
Phenyldiazonium
hydrosulphate
80Physical properties
Name
T pl.,
OS
T boil.,
OS
Solubility
in 100 ml of water
at 25 °С, g
Ka∙1010
Phenol
43
182
9,3
1,1
p-Cresol
35,5
201
2,3
0,07
p-Fluorophenol
48
185
-
5,2
p-Chlorophenol
43
220
2,7
6,3
p-Bromophenol
33
236
-
14
o-Nitrophenol
45
217
600
m-Nitrophenol
96
194
p-Nitrophenol
114
279 diff.
Pyrocatechin
104
246
0.2 volatile
steam
1.35 non-volatile
steam
1.69 non-volatile
steam
46
Resorcinol
110
281
123
600
600
1
3
81Hydrogen bonds
H
O
N
O
O
N
O
O
O
N
H
O
H
O
O
Associated p-nitrophenol
H
H
O
H
O
O
H
+
CO
NO
O
H
H
O
O
The structure of the molecule
..O. H
.
.
+M>-I
.
.
.
.
. .. ..
O
sp2-о Rital
H
2pz-o rital
82Chemical properties
Acid properties of phenols
..
O
R...H
Alcohol
Weaker
acid
.. ....
O H
.. .. ..
O
.. .
+
R O
+ H
.. .
Charge is localized on oxygen
A stronger base
..
....
....
.
.O
O
O
..
..
+H+
..
Phenol
I
II
III
IV
More
strong
charge delocalized
acid
Weaker base
O
Increase in basicity
< ArO < HO < RO
RC
O
O
> ArO H >HO H > RO H Deacidification
RC
O H
83Salt formation
+ H2O
+ NaOH
Phenol, insoluble in water
Oh
D
Acidic
properties
weaken
Phenoxide
sodium, soluble
in water
Oh
A
Acidic
properties
increase
ONa
Oh
ONa
Oh
+ NaHCO3
+ H2CO3
Stronger
acid
Qualitative reaction to phenols.
Formation of colored complexes
compounds with FeCl3:
Phenol - red-violet
Cresol - blue
Resorcinol - dark purple
Salicylic acid - red
The relatively high acidity of phenols is determined by:
1. p-p-conjugation of n-electrons of oxygen and p-electrons of aromatic
kernels. The charge is delocalized.
2. The О-Н bond in phenol is more polarized, because the O atom is bonded to the C atom in
state of sp2 hybridization.
84Phenoxide ion - ambident
nucleophile
O-Alkylation of phenoxide ions.
CH3I
- NaI
Oh
+
NaOH
ONa
CH3
SN2
H3C O SO2 O CH3
O
Methoxybenzene
Anizol
+
- NaOSO2OCH3
C-Alkylation of phenoxide ions.
O CH2 CH CH2
SN
CH2 CH CH2 Br
acetone
O-alkylation
+
ONa
ONa
SN
CH2 CH CH2 Br
CH2CH CH2
benzene
C-alkylation
blocking oxygen
reaction center due to
formation of hydrogen
bonds between the phenolate ion
and excess phenol.
85O-acylation of phenoxide ions (SN).
O
H3C C
..
HO
..
O
H3C C
+
Oh
+
O
..
HO
..
O
CH2 CH3
+
H3C C
H2O HO=+1.5 kcal/mol
H2O HO=-4.6 kcal/mol
O CH2 CH3
O
H3C
NaOH
H2O
Oh
Neutral
moleculeweak
nucleophile
C
Cl
H3C CO O CO CH3 , H+
O
H3C
C
O
+
O Na
SO2Cl
anionic
nucleophile
Phenylacetate
SO2 O
Phenylbenzenesulfonate
86Fries rearrangement
O
H3C
Oh
Oh
C
O
AlCl3, T
CH3
+
SEAr
O
o-Hydroxyacetophenone
Phenyl acetate
H3C
O
p-Hydroxyacetophenone
Reaction scheme
O
OC(O)CH3
+
O
C
H3C
OC(O)CH3
+
CO
AlCl3
H3C
O
C6H5OH
OC(O)CH3 + HO
C
CH3
87Claisen pergrouping
An example of a sigmatropic rearrangement - s-bond displacement
O
CH2
CH
O
CH2
CH2 190-220OC
=
Oh
O
CH
CH2 CH CH2
CH2
H
Allylphenyl ether
Allyloxybenzene
Cyclohexadienone
O CH2 CH CH CH3
220 OS
CH2 CH CH2
2-Allylphenol
Oh
CH HC CH2
CH3
phenyl-(1-but-2-enyl)
ether
1-(but-2-enyloxy)benzene
2-(1-methylallyl)phenol
88Electrophilic substitution SEAr
Oh
Oh
Oh
E
+E+
+
+
o-isomer
H
+
E
p-isomer
SEAr reactions in the phenoxide anion
O
O
O
E
+
E+
H
+
E
H
s-complex
s-complex is a neutral particle,
has increased stability
89Halogenation, SEAr
Highly polar solvent - dissociation of phenol
Oh
+
-H3O
+
O
Oh
Br
H2O
+ 3Br2
phenoxidanion
Br
H2O
+ 3HBr
Br
2,4,6-Tribromophenol
Weakly polar solvent, phenol does not dissociate.
Oh
Oh
Oh
+ Cl2
Cl
OOC
+
CCl4
(74%)
o-Chlorophenol
(26%)
Cl p-Chlorophenol
90Mechanism of bromination of the phenoxide ion
O
O
H
Br2.5OC
Oh
O
Br
Br
Br
Br
-HBr
Br
...
H2O
Br
2,4,6-tribromophenol
Halogenation of phenol in the presence of a strong acid
Oh
Oh
+
br2,
HBr, 5OC
H2O
+
HBr
Completely overwhelmed
phenol dissociation
(81%)
Br
4-bromophenol
91Nitration, SEAr
Oh
Oh
Oh
NO2
20% HNO3, 10OC
+
-H2O
+
o-isomer is separated
during distillation with water
ferry.
NO2
ipso-nitration
Oh
Products
oxidation
Oh
SO3H
HNO3, 0OC
H2SO4 , 100OC
SO3H
Oh
O2N
Oh
SO3H
HNO3,100OC O2N
NO2
ipso substitution
SO3H
NO2
(70%)
92Sulfonation, SEAr
Oh
Oh
SO3H
20OC
+
Oh
H2SO4,
conc.
Kinetic
control
SO3H (51%)
(49%)
Oh
Oh
SO3H
120OC
thermodynamic
control
+
(4%)
SO3H (96%)
93Friedel-Crafts Alkylation, SEAr.
Oh
+
CH3
H2C
C
CH3
KU-2
H3C
H3C
CH3
CH3
CCH3
CH3
Oh
C
CH3
CH3
p-Cresol
2,3-di-tert-Butyl-4-methylphenol
Ionol
Oh
H3C
+
H3C
CH
CH3
HF, 0-8 OC H C
3
Oh
HC
CH3
CH
CH3
Oh
CH
CH3
CH3
2,4,6-Triisopropylphenol
94Friedel-Crafts Acylation, SEAr
Oh
O
+
H3C
C
O
AlCl3; 140OC
O
AlCl3
CH3 Fries rearrangement
Cl
CH3
O
O
+HO
HO
CH3
1-(2-Hydroxyphenyl)ethanone
1-(4-Hydroxyphenyl)ethanone
Oh
+
CH3COOH BF3
20
OC
O
C
HO
CH3
(95%)
95Condensation of phenol with phthalic anhydride, SEAr
A variation of the Friedel-Crafts acylation reaction (A. Bayer).
Oh
HO
O
Oh
C
2
+
O
H2SO4, 120OC
C
O
C
C
O
O
Phenolphthalein
Phthalic
anhydride
Azo combination, SEAr
+
N2Cl
hydrochloric acid
phenyldiazonium
(electrophile)
+
O Na
+
N
N
Oh
4-Phenylazophenol
96Formylation of phenols.
Riemeier-Thiemann reaction.
Oh
Oh
60
Oh
CHO
OC
Cl
+
+ CHCl3 + NaOH CHCl -HO
3
CHCl3OH-
2
(20%)
o-Hydroxybenzaldehyde
CHO
(10%)
+
+ .CCl2
H2O
+ .CCl3
Dichlorocarbene electrophile, not
having a charge.
Carbon has
6 electrons
Formylation of phenols with other reagents
Oh
Oh
+ HCN + HCl
AlCl3
4-Oxybenzalbehyde
benzene, 40
OS
(30%)
CHO
Oh
O
+
H
C
N
CH3
CH3
DMF
Oh
POCl3
DMF, 20
4-Oxybenzalbehyde
OS
(85%)
CHO
97Condensation of phenols with aldehydes and ketones
Oh
Oh
+
H
C
Oh
CH2OH
O NaOH, HO
2
4-Hydroxymethylphenol
+
H
2-Hydroxymethylphenol
reaction mechanism
CH2OH
Oh
O
CH2OH
CH2 O
O
O
"-
C+
H
H
"-
H
NaOH, H2O
O
H
Oh
CH2 O
CH2OH
Oh
O
2
+
H3C
C
HCl
CH3
CH3
HO
C
Oh
CH3
2,2-di(4-hydroxyphenyl)propane
Bisphenol A
98Phenol-formaldehyde resin (L. Backelund, 1909).
The first synthetic high-molecular substance.
Oh
n
+
nHCOH
Oh
20-150OC
NaOH, H2O
Oh
Oh
Bakelite
Oh
n
HO
HO
Oh
99Carboxylation of phenoxide ions.
Kolbe-Schmidt reaction.
ONa
Oxidation.
+
CO2
Na2Cr2O7
COONa
180OC, 5atm
O
Oh
Oh
H2SO4, H2O
O
Oh
OK
O
Oh
+
CO2
p-quinone
250OC, 5atm
OH NaCrO
2
2 7
O
H2SO4, H2O
COOK
Recovery
Oh
Oh
H2, Ni, T, P
Cyclohexanol
100Epoxy resin
n
O
O
+
nCl
CH2
O
epichlorohydrin
CH3
H2C
CHCH2O
O
C
CH3
C
O CH2 CH CH2 O
CH3
Oh
O
CH3
n
CH2CH CH2
O
Elastic material
O
O
H2C
CH2CH CH2
CH CH2
H2N CH2 CH2 NH CH2 CH2 NH2
CH2CH CH2
O
Polymer chain crosslinks, solid material formation
Getting bromide butyl
Butyl bromide, the preparation of which is described in this work, is used in organic synthesis.
) for the synthesis of n-octane:
2CH3CH2CH2CH2Br + 2Na CH3 (CH2) 6CH3 + 2NaBr 2) to obtain butyne-1, which in turn is used as a catalyst in the production of synthetic rubber (condensation of acetylene with formaldehyde): Bromobutane -> Butene -> Dibromobutane -> Butine Properties of n-butyl alcohol CH 3-CH 2-CH 2-CH 2-OH Butyl alcohol (n-butanol) is a representative of monohydric alcohols. Colorless viscous liquid with a characteristic smell of fusel oil. Miscible with organic solvents. Application Butanol is used: · as a solvent in the paint and varnish industry, in the production of resins and plasticizers · modifiers of urea and melamine-formaldehyde resins. · to obtain plasticizers: dibutyl phthalate, tributyl phosphate. · to obtain butyl acetate and butyl acrylate and ethers with glycols. · in the synthesis of many organic compounds. Application as automotive fuel May, but need not, be blended with conventional fuels. The energy of butanol is close to that of gasoline. Butanol can be used in fuel cells as a feedstock for hydrogen production. In 2007, biobutanol began to be sold in the UK as a gasoline additive. Molar mass 74.12 g/mol Density 0.81 g/cm 3 Boiling temperature 117.4º WITH Solubility in water 7.9 g / 100 ml Refractive index 1.399 Methods for obtaining butanol 1)Oxosynthesis from propylene in the presence of HCo(CO) 4at 120-160°С and 20-35 MPa: CH 3 -CH =CH 2+ CO + H 2® CH 3CH 2CH 2CHO + (CH 3)2CHCH =O CH 3(CH 2)3OH + (CH 3)2CHCH 2Oh 2)From propylene in one stage under pressure. 1.0-1.5 MPa (according to Reppe): CH 3 -CH =CH 2+ 3CO + 2H 2O CH 3(CH 2)3OH + (CH 3)2CHCH 2OH+2CO 2
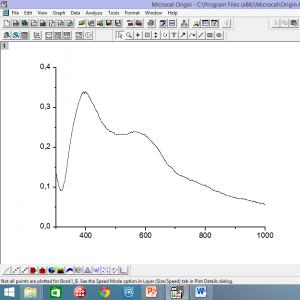
)From acetaldehyde through acetaldol and crotonaldehyde, which is hydrogenated on copper, copper-chromium or nickel catalysts: 2CH 3CHO CH 3CH(OH)CH 2CHO CH 3CH =CHCHO CH 3(CH 2)3Oh )Acetone-butyl fermentation of food. raw materials; the ratio of the resulting products - acetone: n-butanol: ethanol = 3:6:1 Butyl bromide properties Butyl bromide (CH 3CH 2CH 2CH 2Br) is a colorless viscous liquid. Slightly soluble in water, freely soluble in ethanol, acetone, ethers. Molar mass 137.02 g/mol Density 1.299 g/cm 3
Melting point -112.4 º WITH Boiling point 101.6 º WITH Refractive index 1.4398 Receiving method KBr + H 2SO 4® HBr + KHSO 43CH 2CH 2CH 2OH+HBr ® CH 3CH 2CH 2CH 2Br+H 2O butyl alcohol sulfuric bromide The reaction mechanism is nucleophilic substitution (S N 2)
3CH 2CH 2CH 2OH + KBr + H 2SO 4® CH 3CH 2CH 2CH 2Br+KHSO 4+ H 2O Reagents: n-butyl alcohol…………………………. 11.5 ml (9 g) Potassium bromide……………………………. 18.5 g Sulphuric acid ( ρ=1.84) …………………..15 ml Calcium chloride Crockery and equipment: Heating mantle, round bottom flask, reflux condenser, funnel, receiver, thermometer. Synthesis progress Assemble a synthesis setup consisting of a heater, a round bottom flask and a reflux condenser. 17.5 ml of water is poured into a round bottom flask, potassium bromide and butyl alcohol are added, and a reflux condenser is attached. A funnel is inserted into the stock of the refrigerator and 15 ml of concentrated sulfuric acid are poured through it in small portions (2-3 ml each) with constant stirring. Boilers are thrown into the flask and the mixture is carefully heated to a slight boil, boiled for 1 hour. Then the reflux condenser is replaced with a descending condenser (Liebig refrigerator), heating is increased and butyl bromide is distilled off into a receiver with water. The distillation is finished when the oily drops of butyl bromide do not sink to the bottom of the receiver. The contents of the receiver are transferred to a separating funnel, the bottom layer is carefully separated from the water, collecting it in a dry flask. To remove traces of water, butyl bromide is “drained” with anhydrous calcium chloride for 10–15 minutes, periodically shaking the cone. When the liquid becomes transparent, the drying process is considered complete. Butyl bromide is separated from the solid precipitate of calcium chloride by decantation (draining over the edge) into a distillation flask. The apparatus for simple distillation is thoroughly dried beforehand. The receiver is weighed. Butyl bromide is slowly distilled, taking a fraction boiling in the range of 98-103 º C. Determine the volume of the product obtained, measure its refractive index. Exit according to the manual 12.5 g. Observations of the course of the experiment. After the reaction of potassium bromide and n-butanol with sulfuric acid, the mixture separated into two layers: the upper oily layer was light brown and the lower one was transparent. The distillation was carried out until the oily drops stopped dripping to the bottom of the receiver. When dried, the pieces of calcium chloride swelled significantly, which indicated a large amount of water in the resulting product. During distillation, the product became almost transparent. The resulting product was identified by IR spectrum. Based on the spectrum, the following conclusions were made: · There is an intense C-H absorption band in sp 3- hybrid state in the area of 2900 cm -1, indicating the presence - CH 2- hydrocarbon radical · There is an absorption band of 1250 cm -1, characteristic of a class of ether compounds that could be present in the raw product due to possible intermolecular dehydration of the alcohol · Observing a band of medium intensity at 1050 cm -1, indicating the presence of primary alcohol