Optical properties of nanoparticle solutions. Surface plasmon resonance
Surface Plasmon Resonance of Silver Nanoparticles in Lithium Disilicate Glass with Stoichiometric Composition
Institute of Silicate Chemistry named after I. V. Grebenshchikov RAS,
Makarova, St. Petersburg, 199034 Russia
e-mail *****@***ru
Surface plasmon resonance of nanoparticles is a sharp increase in the intensity of absorption and scattering at a certain wavelength of incident light falling into resonance with the natural frequency of oscillations of the electron gas on the surface of the nanoparticle. The parameters of the plasmon resonance are: its magnitude, position in the spectrum, and half-width of the band. They depend on the material, shape, size of the nanoparticle, as well as on the composition of the environment. A study was carried out in which, on the same samples of photostructured (photosensitive) glasses with additives of silver impurity 0.03Ag (wt.%) over 100% and cerium dioxide 0.05CeO2 (wt.%) over 100%, introduced both separately and jointly, the crystallization and optical properties of glass of the stoichiometric composition of lithium disilicate 33.5Li2O 66.5SiO2 (mol.%) were studied:
When exposed to ultraviolet radiation and heat treatment, reducing ions donate electrons to silver ions, converting them into an atomic state. X-ray radiation does not require the introduction of a sensitizer. At an elevated temperature, silver atoms form nanoparticles that serve as crystallization centers for the main nonmetallic phase of lithium disilicate.
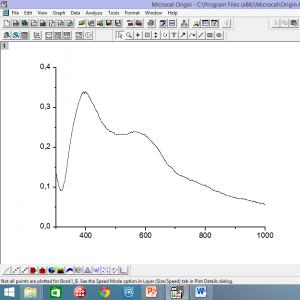
Since the maximum rate of nucleation of lithium disilicate crystals is observed at a temperature of 460 °C, we chose this temperature to study the optical properties of glass. The samples were kept at a temperature of 460°C for 3 hours. Figure 1 shows the dependences of the optical density of the samples, D, on the wavelength for the original glass 1 (without impurities and irradiation); with impurities of silver and cerium dioxide 2; with an admixture of silver 3. Samples 2 and 3 were irradiated for 10 minutes. Heat treatment mode 460 °С 3 hours.
As can be seen from Figure 1, the dependence of the optical density of sample 1 does not have maxima, it gradually decreases. The optical density of the sample with cerium and silver has two maxima: the first is for a wavelength of 310 nm, the second lies at λ = 425 nm, and, finally, the optical density of the sample with silver has only one maximum at λ = 425 nm. Hence, we can conclude that the absorption band at a wavelength of λ = 310 nm is associated with the presence of cerium ions in the glass, and the wavelength λ = 425 corresponds to the plasmon resonance of silver nanoparticles.
Work Conclusions
A comprehensive study was carried out in which, on the same samples of photostructured (photosensitive) glass of the stoichiometric composition, lithium disilicate 33.5Li2O 66.5SiO2 (mol.%) with the addition of a photosensitive impurity of silver (0.03 wt.% over 100%) and wt.% over 100%), introduced both separately and together, the crystallization and optical properties were studied. It has been established that the absorption band at a wavelength of λ = 310 nm is associated with the presence of cerium ions in the glass, and the wavelength λ = 425 corresponds to the plasmon resonance of silver nanoparticles.
The nucleation rate of lithium disilicate on silver particles for a sample depth of 0.52 mm is 500 times higher than the nucleation rate under homogeneous nucleation conditions, which makes it possible to recommend that lithium silicate glass of this composition be used as a photostructured material for obtaining photosensitive glasses and photositalls.
1. A. Crystal nucleation in lithium silicate photosensitive glasses. LAP LAMBERT Academic Publishing. ISBN: 978-3-8454-1285-6. 148s. Project number (24811). LAP LAMBERT Academic Publishing GmbH & Co. KG Dudweiler Landstraße 99, 66123 Saarbrücken Germany. 2011
2. A., V., A., A. Influence of gold nanoparticles on the processes of amorphization and crystallization in photostructurized lithium silicate glass, Fiz. and chem. glass. 2013. V.39. No. 4. pp.513-521.
When electromagnetic radiation interacts with metal nanoparticles, the mobile conduction electrons of the particles are displaced relative to the positively charged metal ions of the lattice. This shift is collective in nature, in which the movement of electrons is consistent in phase. If the particle size is much smaller than the wavelength of the incident light, then the movement of electrons leads to the appearance of a dipole. The result is a force that tends to return the electrons to their equilibrium position. The magnitude of the restoring force is proportional to the magnitude of the displacement, as for a typical oscillator, so we can talk about the presence of a natural frequency of collective oscillations of electrons in a particle. If the oscillation frequency of the incident light coincides with the natural oscillation frequency of free electrons near the surface of a metal particle, there is a sharp increase in the oscillation amplitude of the "electron plasma", the quantum analogue of which is a plasmon. This phenomenon is called surface plasmon resonance (SPR). A peak appears in the light absorption spectrum. For noble metal particles with a size of about 10–100 nm, SPR is observed in the visible region of the spectrum and in the near infrared range. Its position and intensity depend on the size and shape of the nanoparticles and the local dielectric environment. Spherical silver nanoparticles with a diameter of 10–25 nm have an absorption peak near 400–420 nm (Fig. 1a), spherical gold nanoparticles have an absorption peak of 520 nm, and copper (I) oxide nanoparticles have an absorption peak of 450–700 nm.
Nanorods have anisotropic symmetry, and therefore two peaks are observed in the absorption spectrum, corresponding to transverse and longitudinal plasmons. v
near infrared region. Its position is determined by the dimensional factors of the nanorod, namely, by the ratio of length to width.

λ, nm
λ, nm
Fig.1a Optical absorption spectrum of silver nanoparticles
Fig.1b Optical absorption spectrum of rod-like silver nanoparticles
Experimental Processing and presentation of laboratory results
The report must include:
Scheme and equation of the reaction for the synthesis of nanoparticles
Records of solution color change during synthesis
Records of the influence (or lack of influence) of the concentration of the reducing agent and / or stabilizer on the size and stability of the resulting nanoparticles
Absorption spectrum of a solution of nanoparticles
Conclusions on the shape and size of nanoparticles in the synthesized solution
Laboratory work No. 1 Obtaining Ag nanoparticles by the citrate method
This method makes it possible to obtain relatively large silver particles with a diameter of 60–80 nm. The absorption maximum is 420 nm.
Reagents and equipment
Reagents: 0.005M solution of silver nitrate AgNO 3 , sodium citrate Na 3 C 6 H 5 O 7 ∙6H 2 O (1% solution), distilled water.
Equipment: balance, spectrophotometer, quartz cuvettes with an optical path length of 1 cm, 200 ml flasks, 50 ml beakers, heated stirrer, measuring cylinder.
Work order
Prepare a 0.005 M (0.085%) solution of AgNO 3 in water. To do this, dissolve 0.0425 g of the substance in 50 ml of distilled water.
Transfer 25 ml of the prepared solution into a flask and add 100 ml of water.
Prepare a 1% solution of sodium citrate by dissolving 0.5 g of it in 50 ml of water.
Heat 125 ml of the resulting silver nitrate solution to a boil on a hotplate with a stirrer.
As soon as the solution begins to boil, add 5 ml of 1% sodium citrate solution to it.
Heat the solution until the color turns pale yellow.
Leave the solution to cool to room temperature with the stirrer on.
Reduced by boiling, bring the volume of the solution with water to 125 ml.
Read the absorption spectrum of the obtained colloidal solution in the range of 200 - 800 nm. Take water as a reference solution.
Remove the absorption spectrum in a day, a week. Compare the obtained spectra. What can be said about the stability of nanoparticles? What factors determine the stability of nanoparticles obtained by this method? What other ways are known to improve the stability of metal nanoparticles? Why is an aqueous solution of silver nitrate stored in a laboratory in a dark container?
Add dropwise 5 ml of dilute HCl to 5 ml of a solution of the obtained silver nanoparticles. Repeat the experiment with acetic acid CH 3 COOH. Observe the gradual dissolution of silver nanoparticles and the formation of a white precipitate when hydrochloric acid is added and the solution becomes colorless when acetic acid is added. Write down the conclusions, observations and equations of reactions in a notebook.
, polariton , plasmon , nanophotonics Definition plasmon resonance (in the case of nanosized metal structures - localized plasmon resonance) is the excitation of a surface plasmon at its resonant frequency by an external electromagnetic wave. Description
The surface plasmon is not directly related to electromagnetic radiation in the medium adjacent to the metal, since its speed is less than the speed of light. A technique that allows the use of surface plasmons in optics is based on the use of total internal reflection. With total internal reflection, an electromagnetic wave propagates along a surface reflecting light, the speed of which is less than the speed of light and depends on the angle of incidence. If, at a certain angle of incidence, the velocity of this wave coincides with the velocity of a surface plasmon on the metal surface, then the conditions for total internal reflection will be violated, and the reflection will cease to be complete, and a surface plasmon resonance will arise.
In nanoscale metallic systems, collective electronic excitations are modified. The collective electronic excitation of metal nanoparticles, the size of which is smaller than the wavelength of electromagnetic radiation in the environment - a localized surface plasmon - oscillates at a frequency that is ≈3 times lower than the frequency of the bulk plasmon, while the frequency of the surface plasmon is approximately ≈2 times lower than the frequency of the bulk plasmon. plasmon. Due to the small size of the system, the requirement that the velocity of propagation of the excitation and the electromagnetic wave in the external medium should coincide is eliminated, so that the localized surface plasmons are directly related to the radiation. When the frequency of the external field coincides with the frequency of the localized surface plasmon, a resonance arises, which leads to a sharp increase in the field on the surface of the particle and an increase in the absorption cross section.
The properties of localized plasmons critically depend on the shape of the nanoparticles, which makes it possible to tune the system of their resonances for effective interaction with light or elementary quantum systems.
At present, the phenomenon of surface plasmon resonance is widely used in the creation of chemical and biological sensors. Upon contact with biological objects (DNA, viruses, antibodies), plasmonic nanostructures make it possible to increase the intensity of fluorescence signals by more than an order of magnitude; significantly expand the possibilities of detection, identification and diagnostics of biological objects.
- Naimushina Daria Anatolievna
- Perlin E.Yu., Vartanyan T.A., Fedorov A.V. Solid state physics. Optics of semiconductors, dielectrics, metals: Textbook. - St. Petersburg: SPbGU ITMO, 2008. - 216 p.
- Pompa P.P., Martiradonna L. et al. Metal-enhanced fluorescence of colloidal nanocrystals with nanoscale control // Nature Nanotechnology - vol. 1, 2006 - P. 126-130
- Nashchekin A.V. Biosensors based on surface plasmon resonance // Collection of abstracts of sectional reports, poster presentations and reports of participants in the competition of scientific works of young scientists - Second International Forum on Nanotechnology, 2008
The science
Encyclopedic Dictionary of Nanotechnology. - Rusnano. 2010 .
See what "plasmon resonance" is in other dictionaries:
English plasmon resonance) excitation of a surface plasmon at its resonant frequency by an external electromagnetic wave (in the case of nanoscale metal structures, it is called localized plasmon resonance). Description Technical ... Wikipedia
Term nanopharmacology English term nanopharmacology Synonyms Abbreviations Related terms adhesion, gene delivery, antibody, bacteriophage, proteins, biological membrane, hyperthermia, DNA, capsid, quantum dot, kinesin, cell… Encyclopedic Dictionary of Nanotechnology
Molecular-sized gears based on nanotubes ... Wikipedia
Molecular size nanogears Nanotechnology is an interdisciplinary field of fundamental and applied science and technology that deals with a combination of theoretical justification, practical methods of research, analysis and synthesis, as well as ... ... Wikipedia
Molecular size nanogears Nanotechnology is an interdisciplinary field of fundamental and applied science and technology that deals with a combination of theoretical justification, practical methods of research, analysis and synthesis, as well as ... ... Wikipedia
Molecular size nanogears Nanotechnology is an interdisciplinary field of fundamental and applied science and technology that deals with a combination of theoretical justification, practical methods of research, analysis and synthesis, as well as ... ... Wikipedia
In physics, a plasmon is a quasiparticle corresponding to the quantization of plasma oscillations, which are collective oscillations of a free electron gas. Contents 1 Explanation 2 Possible uses ... Wikipedia
In physics, a plasmon is a quasiparticle corresponding to the quantization of plasma oscillations, which are collective oscillations of a free electron gas. Explanation Plasmons play a large role in the optical properties of metals. Light with frequency ... Wikipedia
Gold- (Gold) Gold is a precious metal Gold: cost, samples, rate, buying, varieties of gold Contents >>>>>>>>>>>>>>>> Gold is, definition ... Encyclopedia of the investor