About all RNAs in the world, large and small. Small RNAs and Cancer Small RNA Functions
Scientists believe that the incorrect expression of small RNAs is one of the causes of a number of diseases that very seriously affect the health of many people around the world. Among such diseases are cardiovascular 23 and oncological 24. As for the latter, this is not surprising: cancer testifies to abnormalities in the development of cells and in their fate, and small RNAs play an important role in the corresponding processes. Here is one of the most revealing examples of the enormous effect that small RNAs have on the body in cancer. We are talking about a malignant tumor, which is characterized by the incorrect expression of those genes that act during the initial development of the organism, and not in the postnatal period. It is a type of childhood brain tumor that usually appears before the age of two. Alas, this is a very aggressive form of cancer, and the prognosis is poor even with intensive treatment. The oncological process develops as a result of improper redistribution of genetic material in brain cells. The promoter, which usually causes strong expression of one of the genes encoding proteins, undergoes recombination with a specific small RNA cluster. Then this entire rearranged region is amplified: in other words, many copies of it are created in the genome. Consequently, small RNAs located “downstream” than the displaced promoter are expressed much more strongly than they should. The content of active small RNAs is approximately 150-1000 times higher than normal.
Fig. 18.3. Small RNAs activated by alcohol can bind to messenger RNAs that do not affect the body's resistance to alcohol. But these small RNAs do not bind to messenger RNA molecules that contribute to this resistance. This leads to a relative predominance of the proportion of messenger RNA molecules encoding protein variations associated with alcohol resistance.
This cluster encodes over 40 different small RNAs. Actually, this is generally the largest of such clusters available in primates. It is usually expressed only at an early stage of human development, in the first 8 weeks of an embryo's life. Its strong activation in the infant's brain leads to a catastrophic effect on genetic expression. One of the consequences is the expression of an epigenetic protein that adds modifications to the DNA. This leads to large-scale changes in the entire pattern of DNA methylation, and therefore to abnormal expression of all kinds of genes, many of which must be expressed only when immature brain cells divide during the early stages of the body's development. This is how the cancer program 25 starts in the baby's cells.
Such communication between small RNAs and the epigenetic apparatus of a cell can have a significant effect on other situations when a predisposition to cancer develops in cells. This mechanism probably leads to the fact that the effect of impaired expression of small RNAs is enhanced by altering epigenetic modifications that are transmitted to daughter cells from the mother. This is how a scheme of potentially dangerous changes in the nature of gene expression can be formed.
So far, scientists have not figured out all the stages of the interaction of small RNAs with epigenetic processes, but they still manage to get some hints of what is happening. For example, it turned out that a certain class of small RNAs that increase the aggressiveness of breast cancer targets certain enzymes in messenger RNAs that remove key epigenetic modifications. This alters the pattern of epigenetic modifications in the cancer cell and further disrupts genetic expression 26.
Many forms of cancer are difficult to track in a patient. Oncological processes can take place in hard-to-reach places, which complicates the sampling procedure. In such cases, it is difficult for the doctor to monitor the development of the cancer process and the response to treatment. Often, physicians are forced to rely on indirect measurements - say, a tomographic scan of a tumor. Some researchers believe that small RNA molecules could help create a new method for monitoring tumor development, which also allows studying its origin. When cancer cells die, small RNAs leave the cell when it ruptures. These small junk molecules often form complexes with cellular proteins or are wrapped in fragments of cell membranes. Due to this, they are very stable in body fluids, which means that such RNA can be isolated and analyzed. Since their numbers are small, researchers will have to use highly sensitive analytical methods. However, nothing is impossible here: the sensitivity of nucleic acid sequencing is constantly increasing 27. Published data confirming the promise of this approach in relation to breast cancer 28, ovarian cancer 29 and a number of other cancers. Analysis of small circulating RNAs in lung cancer patients has shown that these RNAs help distinguish between patients with a single pulmonary nodule (not requiring therapy) and patients who develop malignant tumor nodules (requiring treatment) 30.
Destruction of the target mRNA can also occur under the influence of small interfering RNA (siRNA). RNA interference is one of the new revolutionary discoveries in molecular biology, and its authors received the Nobel Prize in 2002 for it. Interfering RNAs are very different in structure from other types of RNA and are two complementary RNA molecules approximately 21-28 nitrogenous bases long, which are connected to each other like strands in a DNA molecule. In this case, two unpaired nucleotides always remain at the edges of each of the siRNA strands. The impact is carried out as follows. When the siRNA molecule is inside the cell, it first binds to a complex with two intracellular enzymes - helicase and nuclease. This complex was named RISC ( RNA- induced silencing complex; silence - eng. be silent, shut up; silencing - this is how the process of "turning off" a gene is called in the English language and special literature). Next, the helicase untwists and separates the siRNA strands, and one of the strands (antisense in structure) in combination with the nuclease specifically interacts with the complementary (strictly corresponding to it) region of the target mRNA, which allows the nuclease to cut it into two parts. The cut sections of the mRNA are further exposed to the action of other cellular RNA nucleases, which further cut them into smaller pieces.
Found in plants and lower animal organisms (insects) siRNAs are an important link in a kind of "intracellular immunity" that makes it possible to recognize and quickly destroy foreign RNA. In the event that an RNA-containing virus has entered the cell, such a defense system will prevent it from multiplying. If the virus contains DNA, the siRNA system will prevent it from producing viral proteins (since the mRNA required for this will be recognized and cut), and using this strategy will slow down its spread throughout the body. The siRNA system was found to be extremely legible: each siRNA will recognize and destroy only its own specific mRNA. Substitution of only one nucleotide within the siRNA leads to a dramatic decrease in the effect of interference. None of the gene blockers known so far has this exceptional specificity for its target gene.
Currently, this method is used mainly in scientific research to identify the functions of various cellular proteins. However, it can potentially also be used to create medicines.
The discovery of RNA interference has given new hope in the fight against AIDS and cancer. It is possible that by using siRNA therapy in conjunction with traditional antiviral and anticancer therapies, it is possible to achieve a potentiation effect, when the two effects lead to a more pronounced therapeutic effect than the simple sum of each of them applied separately.
In order to use the siRNA interference mechanism in mammalian cells for therapeutic purposes, ready-made double-stranded siRNA molecules must be introduced into the cells. However, there are a number of problems that currently do not allow for this in practice, and even more so to create some kind of dosage forms. Firstly, the first echelon of the body's defense acts on them in the blood, enzymes - nucleasesthat cut potentially dangerous and unusual for our body double RNA strands. Secondly, despite their name, small RNAs are still quite long, and, most importantly, they carry a negative electrostatic charge, which makes their passive penetration into the cell impossible. And thirdly, one of the most important questions is how to make siRNA work (or penetrate) only in certain ("sick") cells, without affecting healthy ones? And finally, the problem of size. The optimum size for such synthetic siRNAs is the same 21-28 nucleotides. If you increase its length, cells will respond by producing interferon and reducing protein synthesis. On the other hand, if one tries to use siRNAs smaller than 21 nucleotides, the specificity of its binding to the desired mRNA and the ability to form an RISC complex are sharply reduced. It should be noted that overcoming these problems is critical not only for siRNA therapy, but for gene therapy in general.
Some progress has already been made in their solution. For example, scientists are trying to make siRNA molecules more lipophilic, that is, they can dissolve in the fats that make up the cell membrane, and thus facilitate the penetration of siRNA into the cell. And in order to ensure the specificity of work within only certain tissues, genetic engineers include in their structures special regulatory regions that are activated and start reading the information contained in such a structure (and therefore siRNA, if it is included there), only in certain cells fabrics.
For example, researchers from the San Diego School of Medicine at the University of California (University of California, San Diego School of Medicine) have developed a new efficient delivery system for small interfering RNAs (siRNAs), which suppress the production of certain proteins, into cells. This system should become the basis for a technology for the specific delivery of drugs to various types of cancer. “Small interfering RNAs, which carry out the process of so-called RNA interference, have incredible potential for cancer treatment,” explains Professor Steven Dowdy, who led the study: “and while we still have a lot to do, we have developed the technology delivery of drugs to the population of cells - both primary tumor and metastases, without damaging healthy cells ”.
For many years, Dowdy and his colleagues have studied the anti-cancer potential of small interfering RNAs. However, ordinary siRNAs are tiny, negatively charged molecules that, due to their properties, are extremely difficult to deliver into the cell. To achieve this, the scientists used a short signaling protein PTD (peptide transduction domain). Previously, more than 50 “fusion proteins” were created with its use, in which PTD was combined with tumor suppressor proteins.
However, a simple combination of siRNA with PTD does not lead to the delivery of RNA into the cell: siRNAs are negatively charged, PTDs are positively charged, as a result of which a dense RNA-protein conglomerate is formed that is not transported across the cell membrane. Therefore, the researchers first linked PTD to a protein RNA-binding domain that neutralized the negative charge of siRNA (resulting in a fusion protein called PTD-DRBD). Such an RNA-protein complex already easily passes through the cell membrane and enters the cytoplasm of the cell, where it specifically inhibits the messenger RNAs of proteins that activate tumor growth.
To determine the ability of the PTD-DRBD fusion protein to deliver siRNA into cells, the scientists used a cell line derived from human lung cancer. After treatment of cells with PTD-DRBD-siRNA, it was found that tumor cells are most susceptible to siRNA, while in normal cells (T cells, endothelial cells and embryonic stem cells were used as controls), where there was no increased production of oncogenic proteins, no toxic effects were observed.
This method can be subjected to various modifications, using different siRNAs to suppress different tumor proteins - not only overproduced, but also mutant. It is also possible to modify the therapy in the case of recurrent tumors, which usually become resistant to chemotherapy drugs due to new mutations.
Oncological diseases are highly variable, and the molecular characteristics of tumor cell proteins are individual for each patient. The authors of the work believe that in this situation, the use of small interfering RNAs is the most rational approach to therapy.
In a living cell, the flow of information between the nucleus and the cytoplasm never dries up, however, understanding all its “eddies” and deciphering the information encoded in it is a truly titanic task. One of the most important breakthroughs in biology of the last century can be considered the discovery of informational (or matrix) RNA (mRNA or mRNA) molecules, which serve as intermediaries that transfer informational "messages" from the nucleus (from chromosomes) into the cytoplasm. The decisive role of RNA in protein synthesis was predicted back in 1939 in the work of Torbjorn Kaspersson ( Torbjörn Caspersson), Jean Brachet ( Jean brachet) and Jack Schultz ( Jack schultz), and in 1971 George Marbeis ( George marbaix) started the synthesis of hemoglobin in frog oocytes by injecting the first isolated rabbit messenger RNA encoding this protein.
In 1956-1957, in the Soviet Union, A. N. Belozersky and A. S. Spirin independently proved the existence of mRNA, and also found out that the bulk of RNA in a cell is by no means matrix, but ribosomal RNA (rRNA). Ribosomal RNA - the second "main" type of cellular RNA - forms the "skeleton" and functional center of ribosomes in all organisms; it is rRNA (and not proteins) that regulates the main stages of protein synthesis. At the same time, the third "main" type of RNA was described and studied - transport RNA (tRNA), which in combination with two others - mRNA and rRNA - form a single protein-synthesizing complex. According to the rather popular hypothesis of the "RNA world", it was this nucleic acid that lay at the very origins of life on Earth.
Due to the fact that RNA is much more hydrophilic than DNA (due to the replacement of deoxyribose with ribose), it is more labile and can move relatively freely in the cell, and therefore deliver short-lived replicas of genetic information (mRNA) to the place where it begins protein synthesis. However, it is worth noting the "inconvenience" associated with this - RNA is very unstable. It is much worse than DNA, it is stored (even inside the cell) and degrades at the slightest change in conditions (temperature, pH). In addition to "intrinsic" instability, a large contribution belongs to ribonucleases (or RNases) - a class of RNA-cleaving enzymes that are very stable and "ubiquitous" - even the skin of the experimenter's hands contains enough of these enzymes to negate the whole experiment. Because of this, working with RNA is much more difficult than with proteins or DNA - the latter can generally be stored for hundreds of thousands of years with little or no damage.
Fantastic accuracy at work, tridistilate, sterile gloves, disposable laboratory glassware - all this is necessary to prevent RNA degradation, but compliance with such standards was not always possible. Therefore, for a long time, the short "fragments" of RNA, which inevitably contaminated solutions, were simply ignored. However, over time, it became clear that, despite all efforts to maintain the sterility of the working area, the "debris" naturally continued to be found, and then it turned out that there are always thousands of short double-stranded RNAs in the cytoplasm that perform quite specific functions and are absolutely necessary for normal development cells and organism.
RNA interference principle
Pharmacists have also become interested in the possibility of using siRNA, since the ability of directed regulation of the work of individual genes promises unheard-of prospects in the treatment of many diseases. Small size and high specificity of action promise high efficiency and low toxicity of siRNA-based drugs; however solve the problem delivery siRNA for sick cells in the body has not yet succeeded - the reason is the fragility and fragility of these molecules. And although now dozens of teams are trying to find a way to direct these "magic bullets" right on target (inside diseased organs), they have not yet achieved visible success. Besides this, there are other difficulties. For example, in the case of antiviral therapy, the high selectivity of the action of siRNA can be a "disservice" - since viruses mutate rapidly, the modified strain will very quickly lose sensitivity to the siRNA selected at the beginning of therapy: it is known that the replacement of only one nucleotide in siRNA leads to a significant decrease in interference effect.
At this point it is worth recalling again - siRNAs were discovered only in plants, invertebrates and unicellular; although protein homologues for RNA interference (Dicer, RISC complex) are also present in higher animals, siRNAs were not detected by conventional methods. What was the surprise when artificially introduced synthetic siRNA analogs caused a strong specific dose-dependent effect in mammalian cell cultures! This meant that in vertebrate cells, RNA interference was not replaced by more complex immune systems, but evolved along with organisms, turning into something more "advanced". Consequently, in mammals it was necessary to look not for exact analogs of siRNA, but for their evolutionary successors.
Player # 2 - microRNA
Indeed, on the basis of an evolutionarily rather ancient mechanism of RNA interference in more developed organisms, two specialized systems for controlling the work of genes appeared, each using its own group of small RNAs - microRNA (microRNA) and piRNA (piRNA, Piwi-interacting RNA). Both systems appeared in sponges and coelenterates and evolved along with them, displacing siRNA and the mechanism of “naked” RNA interference. Their role in providing immunity is reduced, since this function was taken over by more advanced mechanisms of cellular immunity, in particular, the interferon system. However, this system is so sensitive that it also responds to siRNA itself: the appearance of small double-stranded RNAs in mammalian cells triggers an “alarm” (activates the secretion of interferon and causes the expression of interferon-dependent genes, which blocks all translation processes entirely). In this regard, the mechanism of RNA interference in higher animals is mediated mainly by microRNA and piRNA - single-stranded molecules with a specific structure that are not detected by the interferon system.
As the genome became more complex, microRNAs and piRNAs became increasingly involved in the regulation of transcription and translation. Over time, they developed into an additional, precise and subtle system of genome regulation. Unlike siRNAs, miRNAs and piRNAs (discovered in 2001, see Box 3) are not produced from foreign double-stranded RNA molecules, but are initially encoded in the host genome.
Meet: microRNA
The miRNA precursor is transcribed from both strands of genomic DNA by RNA polymerase II, as a result of which an intermediate form appears - pri-miRNA - carrying the features of ordinary mRNA - m 7 G-cap and polyA-tail. In this precursor, a loop is formed with two single-stranded "tails" and several unpaired nucleotides in the center (Fig. 3). Such a loop undergoes two-stage processing (Fig. 4): first, the Drosha endonuclease cuts off single-stranded RNA “tails” from the hairpin, after which the cut hairpin (pre-microRNA) is exported into the cytoplasm, where it is recognized by Dicer, who makes two more cuts (a double-stranded region is cut out) color-coded in Fig. 3). In this form, mature microRNA, similarly to siRNA, is included in the RISC complex.
Figure 3. Structure of a double-stranded microRNA precursor molecule. Main features: the presence of conservative sequences that form a hairpin; the presence of a complementary copy (microRNA *) with two "extra" nucleotides at the 3 'end; a specific sequence (2–8 bp) that forms a recognition site for endonucleases. The microRNA itself is highlighted in red - this is what Dicer cuts out.
The mechanism of action of many miRNAs is similar to the action of siRNA: a short (21–25 nucleotides) single-stranded RNA in the RISC protein complex binds with high specificity to a complementary region in the 3 'untranslated region of the target mRNA. Binding results in the cleavage of mRNA by the Ago protein. However, the activity of microRNA (compared to siRNA) is already more differentiated - if the complementarity is not absolute, the target mRNA may not degrade, but only be reversibly blocked (there will be no translation). The same RISC complex can also use artificially introduced siRNA. This explains why siRNAs made by analogy with protozoa are also active in mammals.
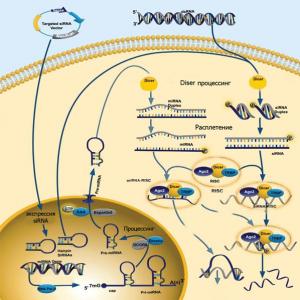
Thus, we can supplement the illustration of the mechanism of action of RNA interference in higher (bilaterally symmetric) organisms by combining in one figure the scheme of action of microRNA and biotechnologically introduced siRNAs (Fig. 5).

Figure 5. Generalized scheme of action of artificial microRNA and siRNA (artificial siRNAs are introduced into the cell using specialized plasmids - targeting siRNA vector).
Functions of microRNA
Physiological functions of microRNAs are extremely diverse - in fact, they are the main non-protein regulators of ontogenesis. miRNAs do not cancel, but supplement the "classical" scheme of gene regulation (inducers, suppressors, chromatin compaction, etc.). In addition, the synthesis of miRNAs themselves is regulated in a complex way (certain pools of miRNAs can be turned on by interferons, interleukins, tumor necrosis factor α (TNF-α), and many other cytokines). As a result, an amazingly complex and flexible multi-level network of tuning an "orchestra" of thousands of genes emerges, but this does not end there either.
microRNAs are more “universal” than siRNAs: “ward” genes do not have to be 100% complementary - regulation is carried out even with partial interaction. Today, one of the hottest topics in molecular biology is the search for microRNAs that act as alternative regulators of known physiological processes. For example, miRNAs involved in the regulation of the cell cycle and apoptosis in plants, Drosophila and nematodes have already been described; in humans, microRNAs regulate the immune system and the development of hematopoietic stem cells. The use of technologies based on biochips (micro-array screening) has shown that entire pools of small RNAs are turned on and off at different stages of cell life. For biological processes, dozens of specific microRNAs have been identified, the expression level of which changes thousands of times under certain conditions, emphasizing the exceptional controllability of these processes.
Until recently, it was believed that microRNAs only suppress - in whole or in part - the work of genes. Recently, however, it turned out that the effect of microRNA can be radically different depending on the state of the cell! In an actively dividing cell, microRNA, by binding to the complementary sequence in the 3 'region of mRNA, inhibits protein synthesis (translation). However, in a state of rest or stress (for example, when growing in a poor environment), the same event leads to the exact opposite effect - an increase in the synthesis of the target protein!
Evolution of microRNA
The number of microRNA varieties in higher organisms has not yet been fully established - according to some data, it exceeds 1% of the number of protein-coding genes (in humans, for example, they speak of 700 microRNAs, and this number is constantly growing). miRNAs regulate the activity of about 30% of all genes (targets for many of them are not yet known), and there are both ubiquitous and tissue-specific molecules - for example, one such important pool of miRNAs regulates the maturation of blood stem cells.
The wide expression profile in different tissues of different organisms and the biological prevalence of microRNAs indicate an evolutionary ancient origin. For the first time miRNAs were found in nematodes, and for a long time later it was believed that these molecules appear only in sponges and coelenterates; however, they were later discovered in unicellular algae as well. Interestingly, as organisms become more complex, the amount and heterogeneity of the microRNA pool also increases. This indirectly indicates that the complexity of these organisms is provided, in particular, by the functioning of microRNA. The possible evolution of microRNAs is shown in Figure 6.

Figure 6. Diversity of microRNAs in different organisms. The higher the organization of the organism, the more microRNA is found in it (the number in brackets). Species are highlighted in red, in which single microRNA.
A clear evolutionary relationship can be drawn between siRNA and microRNA, based on the following facts:
- the action of both types is interchangeable and is mediated by homologous proteins;
- siRNAs introduced into mammalian cells specifically “turn off” the desired genes (despite some activation of interferon defense);
- microRNAs are found in more and more ancient organisms.
These and other data suggest the origin of both systems from a common "ancestor". It is also interesting to note that "RNA" immunity as an independent precursor of protein antibodies confirms the theory of the origin of the first forms of life based on RNA, not proteins (recall that this is the favorite theory of Academician A.S. Spirin).
The further, the more confusing. Player # 3 - piRNA
While there were only two "players" in the arena of molecular biology - siRNA and microRNA - the main "purpose" of RNA interference seemed completely clear. Indeed: a set of homologous short RNAs and proteins in different organisms performs similar actions; as organisms become more complex, functionality becomes more complex.
However, in the process of evolution, nature created another, evolutionarily the most recent and highly specialized system based on the same successful principle of RNA interference. We are talking about piRNA (piRNA, from Piwi-interaction RNA).
The more complex the genome is organized, the more developed and adapted the organism (or vice versa? ;-). However, the increase in genome complexity also has a downside: the complex genetic system becomes unstable... This leads to the need for mechanisms responsible for maintaining the integrity of the genome - otherwise the spontaneous "mixing" of DNA will simply disable it. Mobile genetic elements ( IGE) - one of the main factors of genome instability - are short unstable regions that can be autonomously transcribed and migrated throughout the genome. The activation of such mobile elements leads to multiple DNA breaks in chromosomes, fraught with lethal consequences.
The number of SHEs increases nonlinearly with the size of the genome, and their activity must be contained. For this, animals, starting with coelenterates, use the same phenomenon of RNA interference. This function is also performed by short RNAs, however, not the ones mentioned above, but their third type, piRNAs.
PiRNA "portrait"
PiRNA functions
The main function of piRNA is to suppress MGE activity at the level of transcription and translation. It is believed that piRNAs are active only during embryogenesis, when unpredictable shuffling of the genome is especially dangerous and can lead to the death of the embryo. This is logical - when the immune system has not yet started working, the cells of the embryo need some simple but effective protection. The embryo is reliably protected from external pathogens by the placenta (or egg shell). But besides this, defense is also needed from endogenous (internal) viruses, primarily MGE.
This role of piRNA has been confirmed by experience - “knockout” or mutations of the Ago3, Piwi or Aub genes lead to serious developmental disorders (and a sharp increase in the number of mutations in the genome of such an organism), and also cause infertility due to impaired development of germ cells.
Distribution and evolution of piRNA
The first piRNAs are found already in anemones and sponges. Plants apparently took a different path - Piwi proteins were not found in them, and the role of “muzzle” for transposons is played by endonuclease Ago4 and siRNA.
In higher animals, including humans, the piRNA system is very well developed, but it can be found only in embryonic cells and in the amniotic endothelium. Why the distribution of piRNAs in the body is so limited remains to be seen. It can be assumed that, like any powerful weapon, piRNAs are beneficial only in very specific conditions (during fetal development), and in the adult body, their activity will do more harm than good. Still, the number of piRNAs is an order of magnitude larger than the number of known proteins, and the nonspecific effects of piRNAs in mature cells are difficult to predict.
| siRNA | microRNA | piRNA | |
|---|---|---|---|
| Spread | Plants, Drosophila, C. elegans... Not found in vertebrates | Eukaryotes | Embryonic animal cells (starting with coelenterates). No protozoa and plants |
| Length | 21-22 nucleotides | 19-25 nucleotides | 24-30 nucleotides |
| Structure | Double-stranded, 19 complementary nucleotides each and two unpaired nucleotides at the 3 'end | Single-stranded complex structure | Single-stranded complex structure. U at the 5'-end, 2'- O-methylated 3'-end |
| Processing | Dicer-addicted | Dicer-addicted | Dicer-independent |
| Endonuclease | Ago2 | Ago1, Ago2 | Ago3, Piwi, Aub |
| Activity | Degradation of complementary mRNA, acetylation of genomic DNA | Degradation or inhibition of translation of the target mRNA | Degradation of mRNA encoding MGE, regulation of MGE transcription |
| Biological role | Antiviral immune defense, suppression of the activity of own genes | Regulation of gene activity | Suppression of MGE activity during embryogenesis |
Conclusion
In conclusion, I would like to give a table illustrating the evolution of the protein apparatus involved in RNA interference (Fig. 9). It can be seen that protozoa have the most developed siRNA system (protein families Ago, Dicer), and as organisms become more complex, the emphasis is shifted to more specialized systems - the number of protein isoforms for microRNA (Drosha, Pasha) and piRNA (Piwi, Hen1) increases. In this case, the variety of enzymes that mediate the action of siRNA decreases.

Figure 9. Diversity of proteins involved in RNA interference (the numbers indicate the amount of proteins in each group). In blue the elements characteristic of siRNA and microRNA are highlighted, and red - protein andassociated with piRNA.
The phenomenon of RNA interference has already begun to be used by the simplest organisms. Based on this mechanism, nature has created a prototype of the immune system, and as organisms become more complex, RNA interference becomes an indispensable regulator of genome activity. Two different mechanisms plus three types of short RNAs ( cm. tab. 1) - as a result, we see thousands of subtle regulators of various metabolic and genetic pathways. This striking picture illustrates the versatility and evolutionary adaptation of molecular biological systems. Short RNAs again prove that there are no "little things" inside the cell - there are only small molecules, the full significance of the role of which we are just beginning to understand.
(True, such a fantastic complexity speaks rather of the fact that evolution is "blind" and operates without a pre-approved "general plan";
), preventing the translation of mRNA on ribosomes into the protein encoded by it. Ultimately, the effect of small interfering RNAs is identical to that if gene expression simply decreased.
Small interfering RNAs were discovered in 1999 by David Baulcombe's group in the UK as a component of the post-transcriptional gene silencing system in plants. PTGS, en: post-transcriptional gene silencing). The group published the findings in the journal Science.
Double-stranded RNAs can enhance gene expression by a mechanism called RNA-dependent gene activation (eng. RNAa, small RNA-induced gene activation). It has been shown that double-stranded RNAs complementary to the promoters of target genes cause the activation of the corresponding genes. RNA-dependent activation with the introduction of synthetic double-stranded RNAs has been shown in human cells. It is not known whether a similar system exists in the cells of other organisms.
Providing the ability to turn off essentially any gene at will, RNA interference based on small interfering RNAs has generated tremendous interest in basic and applied biology. The number of large-scale RNA interference tests to identify important genes in biochemical pathways is constantly growing. Since disease progression is also driven by gene activity, it is expected that, in some cases, gene shutdown by small interfering RNA may have a therapeutic effect.
However, the application of RNA interference based on small interfering RNAs to animals, and in particular to humans, faces many difficulties. Experiments have shown that the efficiency of small interfering RNAs is different for different types of cells: some cells easily respond to the effects of small interfering RNAs and demonstrate a decrease in gene expression, while in others this is not observed, despite effective transfection. The reasons for this phenomenon are still poorly understood.
The results of the first phase of trials of the first two therapeutic drugs acting on the RNA interference mechanism (intended for the treatment of macular degeneration), published at the end of 2005, show that drugs based on small interfering RNAs are easily tolerated by patients and have acceptable pharmacokinetic properties.
Preliminary clinical trials of small interfering RNAs targeting the Ebola virus indicate that they may be effective for post-exposure prophylaxis of the disease. This drug allowed the entire group of experimental primates who received a lethal dose of the Zaire Ebolavirus to survive
SiRNAs are 21-25 bp in length and are derived from dsRNAs. The source of such RNAs can be viral infections, genetic constructs introduced into the genome, long hairpins in transcripts, and bidirectional transcription of mobile elements.
dsRNAs are cut by Dicer RNase into 21-25 bp fragments. with 3 "ends protruding by 2 nucleotides, after which one of the strands is part of the RISC and directs the cleavage of homologous RNAs. RISC contains siRNAs corresponding to both plus and minus strands of dsRNA. siRNAs do not have their own genes and represent are fragments of longer RNAs.siRNAs direct the cleavage of the target RNA, since they are completely complementary to it.In plants, fungi and nematodes, RNA-dependent RNA polymerases are involved in the process of suppressing gene expression, for which siRNAs also serve as primers (seeds for the synthesis of new RNA The resulting dsRNA is cut by Dicer, new siRNAs are formed, which are secondary, thus amplifying the signal. 
RNA interference

In 1998, Craig C. Mello and Andrew Fire published in Nature, which stated that double-stranded RNA (dsRNA) can suppress gene expression. Later it turned out that the active principle in this process is short single-stranded RNAs. The mechanism for suppressing gene expression using these RNAs is named
RNA interference as well as RNA silencing. This mechanism has been found in all large taxa of eukaryotes: vertebrates and invertebrates, plants and fungi. In 2006, the Nobel Prize was received for this discovery.
Suppression of expression can occur at the transcriptional level or post-transcriptionally. It turned out that in all cases a similar set of proteins and short (21-32 bp) RNAs are required.
siRNAs regulate gene activity in two ways. As discussed above, they direct the cleavage of target RNAs. This phenomenon is called "suppression" ( quelling) in mushrooms, " post-translational gene silencing"in plants and" RNA interference
"in animals. siRNAs 21-23 bp in length are involved in these processes. Another type of action is siRNAs capable of suppressing the transcription of genes containing homologous siRNA sequences. This phenomenon was named transcriptional gene silencing
(TGS) and is found in yeast, plants and animals. siRNAs also direct DNA methylation, resulting in heterochromatin formation and transcriptional repression. TGS is best studied in the yeast S. pombe, where siRNAs have been found to be inserted into a RISC-like protein complex called RITS. In his case, as in the case of RISC, siRNA interacts with a protein of the AGO family. It is likely that siRNA is able to target this complex to a gene that contains a homologous siRNA fragment. After that, RITS proteins recruit methyltransferases, as a result of which heterochromatin is formed in the locus encoding the siRNA target gene, and active gene expression is terminated. 
Role in cellular processes
 What is the significance of siRNA in the cell?
What is the significance of siRNA in the cell?
siRNAs are involved in cell defense against viruses, repression of transgenes, regulation of some genes, and formation of centromeric heterochromatin. An important function of siRNA is the suppression of the expression of mobile genetic elements. Such suppression can occur both at the transcriptional level and posttranscriptionally.
The genome of some of the viruses consists of DNA, while others are made up of RNA, and the RNA of viruses can be either single- or double-stranded. The process of cutting foreign (viral) mRNA itself in this case occurs in the same way as described above, that is, by activating the RISC enzyme complex. However, for greater efficiency, plants and insects have invented a unique way to enhance the protective effect of siRNA. By attaching to the mRNA strand, the siRNA site can, using the DICER enzyme complex, first complete the second mRNA strand, and then cut it at different places, thus creating a variety of "secondary" siRNAs. They, in turn, form RISC and conduct mRNA through all the stages discussed above, up to its complete destruction. Such "secondary" molecules will be able to specifically bind not only to the region of the viral mRNA to which the "primary" molecule was directed, but also to other regions, which sharply enhances the effectiveness of cellular defense.
Thus, in plants and lower animal organisms, siRNAs are an important link in a kind of "intracellular immunity", which makes it possible to recognize and quickly destroy foreign RNA. In the event that an RNA-containing virus penetrates the cell, such a defense system will prevent it from multiplying. If the virus contains DNA, the siRNA system will prevent it from producing viral proteins (since the mRNA required for this will be recognized and cut), and using this strategy will slow down its spread throughout the body.
In mammals, unlike insects and plants, another defense system works. When a foreign RNA enters a "mature" (differentiated) mammalian cell, the length of which is more than 30 bp, the cell begins to synthesize interferon. Interferon, by binding to specific receptors on the cell surface, is able to stimulate a whole group of genes in the cell. As a result, several types of enzymes are synthesized in the cell that inhibit protein synthesis and break down viral RNAs. In addition, interferon can act on neighboring, not yet infected cells, thereby blocking the possible spread of the virus.
As you can see, both systems are similar in many ways: they have a common goal and "methods" of work. Even the names "interferon" and "(RNA) interference" come from a common root. But they also have one very significant difference: if interferon, at the first signs of an invasion, simply "freezes" the work of the cell, preventing (just in case) the production of many, including "innocent" proteins in the cell, then the siRNA system is extremely legible : each siRNA will recognize and destroy only its own specific mRNA. Substitution of just one nucleotide within the siRNA leads to a sharp decrease in the effect of interference ... None of the gene blockers known so far has this exceptional specificity for its target gene.
The discovery of RNA interference has given new hope in the fight against AIDS and cancer. Perhaps, by using siRNA therapy in conjunction with traditional antiviral therapy, it is possible to achieve a potentiation effect, when the two effects lead to a more pronounced therapeutic effect than the simple sum of each of them applied separately.
In order to use the siRNA interference mechanism in mammalian cells, ready-made double-stranded siRNA molecules must be introduced into the cells. The optimal size of such synthetic siRNAs is the same 21-28 nucleotides. If you increase its length, cells will respond by producing interferon and reducing protein synthesis. Synthetic siRNAs can enter both infected and healthy cells, and a decrease in protein production in uninfected cells will be highly undesirable. On the other hand, if one tries to use siRNAs smaller than 21 nucleotides, the specificity of its binding to the desired mRNA and the ability to form an RISC complex are sharply reduced.
If siRNA can be delivered in one way or another that has the ability to bind to some part of the HIV genome (which, as is known, consists of RNA), one can try to prevent its integration into the host cell's DNA. In addition, scientists are developing ways of influencing the different stages of HIV reproduction in an already infected cell. The latter approach will not provide a cure, however, it can significantly reduce the rate of reproduction of the virus and give the cornered immune system a chance to "rest" from the viral attack, and try to deal with the remnants of the disease itself. In the figure, the two stages of HIV multiplication in the cell that scientists hope can be blocked by siRNA are marked with red crosses (stages 4-5 - the insertion of the virus into the chromosome, and stages 5-6 - the assembly of the virus and exit from the cell). 
To date, however, all of the above applies only to the field of theory. In practice, siRNA therapy encounters difficulties that scientists have not yet been able to avoid. For example, in the case of antiviral therapy, it is the high specificity of siRNA that can play a cruel joke: as you know, viruses have the ability to quickly mutate, i.e. change the composition of their nucleotides. HIV has especially succeeded in this, the frequency of changes in which is such that a person who has become infected with one subtype of the virus, after a few years, can be distinguished by a completely different subtype. In this case, the modified HIV strain will automatically become insensitive to the siRNA selected at the beginning of therapy.
Aging and carcinogenesis
 Like any epigenetic factor, siRNAs affect the expression of genes that silence. Now there are works that describe experiments to turn off genes associated with tumors. Genes are knock-down by siRNA. For example, Chinese scientists used siRNA to turn off the gene for transcription factor 4 (TCF4), the activity of which is responsible for Pitt-Hopkins syndrome (a very rare genetic disease characterized by mental retardation and episodes of hyperventilation and apnea) and other mental illnesses. In this work, we studied the role of TCF4 in gastric cancer cells. Ectopic expression of TCF4 reduces cell growth in gastric cancer cell lines, and siRNA deactivation of the TCF4 gene increases cell migration. Thus, it can be concluded that epigenetic switch-off (silencing) of the TCF4 gene plays an important role in tumor formation and development.
Like any epigenetic factor, siRNAs affect the expression of genes that silence. Now there are works that describe experiments to turn off genes associated with tumors. Genes are knock-down by siRNA. For example, Chinese scientists used siRNA to turn off the gene for transcription factor 4 (TCF4), the activity of which is responsible for Pitt-Hopkins syndrome (a very rare genetic disease characterized by mental retardation and episodes of hyperventilation and apnea) and other mental illnesses. In this work, we studied the role of TCF4 in gastric cancer cells. Ectopic expression of TCF4 reduces cell growth in gastric cancer cell lines, and siRNA deactivation of the TCF4 gene increases cell migration. Thus, it can be concluded that epigenetic switch-off (silencing) of the TCF4 gene plays an important role in tumor formation and development.
According to research at the Department of Oncology, the Albert Einstein Cancer Center, led by Leonard H. Augenlicht, siRNA is involved in turning off the HDAC4 gene, which causes inhibition of colon cancer growth, apoptosis, and increased p21 transcription. HDAC4 is a histone deacetylase that is tissue specific, inhibits cell differentiation and its expression is suppressed during the process of cell differentiation. The work showed that HDAC4 is an important regulator of colon cell proliferation (which is important in the cancer process), and it, in turn, is regulated by siRNA.
The Department of Pathology, Nara Medical University School of Medicine in Japan conducted research on prostate cancer. Replicative cell aging is a barrier against uncontrolled division and carcinogenesis. Short-lived dividing cells (TACs) are part of the prostate cell population from which tumors develop. Japanese scientists have studied the reasons why these cells overcome aging. JunB siRNA was transfected into prostate cells in culture. In these cells, an increased level of expression of p53, p21, p16, and pRb is observed, which is detected during aging. Cells in culture that showed reduced p16 levels were used for the next step. Re-transfection of siRNA into TAC allowed cells to avoid senescence when p16 / pRb was inactivated. In addition, the silencing of the junB proto-oncogene by the junB siRNA induces cell invasion. Based on this, it was concluded that junB is an element for p16 and promotes cellular senescence, which prevents TAC malignancy. Thus, junB is a regulator of prostate carcinogenesis and may be a target for therapeutic intervention. And its activity can be regulated using siRNA.
There are a lot of such studies. Nowadays siRNA is not only an object, but also an instrument in the hands of a researcher-doctor, biologist, oncologist, gerontologist. The study of the relationship of siRNA with oncological diseases, with the expression of age-associated genes is the most important task for science. Very little time has passed since the discovery of siRNA, and how many interesting studies and publications related to them have appeared. There is no doubt that their study will become one of the steps of mankind to defeat cancer and aging ...