Big Deals of Small Molecules: How Small RNAs Orchestrate Bacterial Genes. Small RNAs and Cancer Small interfering RNAs do not include
Article for the competition "bio / mol / text": In recent years, RNA - and especially its "non-classical" varieties - has attracted the attention of biologists around the world. It turned out that regulation with the help of noncoding RNAs is widespread - from viruses and bacteria to humans. The study of the diversity of small bacterial RNA regulators clearly showed their important role both in intermediate metabolism and in adaptive responses. This article describes the types of small RNA bacteria and the regulatory mechanisms carried out with their help. Special emphasis is placed on the role of these molecules in the vital activity of bacterial agents that cause especially dangerous infections.
RNA: more than just a copy of DNA
From school, most of the readers of this site are familiar with the basic mechanisms of a living cell. In the course of biology, starting with Mendel's laws and ending with cutting-edge projects on genome sequencing, the idea of \u200b\u200bthe main genetic program for the development of an organism, known to professional biologists as central dogma of molecular biology... It says that the DNA molecule acts as a carrier and custodian of genetic information, which, through an intermediary - messenger RNA (mRNA), and with the participation of ribosomal (rRNA) and transport RNA (tRNA) - is realized in the form of proteins. The latter determine the species and individual phenotype.
This state of affairs and the assignment of RNA to the role of a minor participant in the molecular spectacle persisted in the scientific community until the 80s of the last century. A closer look at RNA was made by the work of T. Chek, who showed that RNA can act as a catalyst for chemical reactions. Previously, it was believed that the acceleration of chemical processes in the cell is the prerogative of enzymes that have an exclusively protein nature. The discovery of catalytic activity in RNA had far-reaching consequences - together with the earlier theoretical works of K. Woese and, it allowed to draw a possible picture of prebiotic evolution on our planet. The fact is that since the discovery of the function of a carrier of genetic information in DNA, the dilemma about what appeared in the course of evolution earlier - DNA or the protein necessary for DNA reproduction - seemed almost as philosophical (that is, pointless) as the question about the primacy of the appearance of a chicken or an egg. After T. Chek's discovery, the solution took on quite real outlines - a molecule was found that possesses the properties of both an information carrier and a biocatalyst (albeit in an embryonic form). Over time, these studies have grown into a whole direction in biology, studying the emergence of life through the prism of the so-called "RNA world".
So it became obvious that the ancient world of RNA could have been related to the origin and flowering of primary life. Nevertheless, it does not automatically follow from this that RNA in modern organisms is not an archaism adapted to the needs of intracellular molecular systems, but a really important member of the molecular ensemble of the cell. Only the development of molecular methods - in particular, the sequencing of nucleic acids - showed that RNAs are truly indispensable in the cell, and not only in the form of the canonical trinity of "mRNA, rRNA, tRNA". Already the first extensive data on DNA sequencing pointed to the fact that at first seemed difficult to explain - most of it turned out to be non-coding - that is, it does not carry information about protein molecules or "standard" RNA. Of course, this can be partially attributed to "genetic garbage" - "off" or having lost their function to fragments of the genome. But preserving that much "dowry" for biological systems that are trying to spend energy economically, it seems illogical.
Indeed, more detailed and subtle research methods have made it possible to discover a whole class of RNA regulators of gene expression that partially fill the intergenic space. Before reading the complete genome sequences of eukaryotes in the roundworm C. elegans microRNAs were isolated - molecules of short length (about 20 nucleotides), which can specifically bind to mRNA regions according to the principle of complementarity. It is easy to guess that in such cases it is no longer possible to read information about the encoded proteins from the mRNA: the ribosome simply cannot “run” over such a region that has suddenly become double-stranded. This mechanism of suppressing gene expression, called RNA interference, has already been disassembled on the "biomolecule" in sufficient detail. To date, thousands of microRNA and other noncoding RNA molecules (piRNA, snoRNA, nanoRNA, etc.) have been discovered. In eukaryotes (including humans), they are located in intergenic regions. Their important role in cell differentiation, carcinogenesis, immune response and other processes and pathologies has been established.
Small RNAs - Trojan Horse for Bacterial Proteins
Despite the fact that noncoding protein RNAs in bacteria were discovered much earlier than the first analogous regulators in eukaryotes, their role in the metabolism of bacterial cells has long been veiled to the scientific community. This is understandable - traditionally, the bacterial cell was considered a more primitive and less mysterious structure for the researcher, the complexity of which cannot be compared with the accumulation of structures in a eukaryotic cell. Moreover, in bacterial genomes the content of noncoding information is only a few percent of the total DNA length, reaching a maximum of 40% in some mycobacteria. But, given that microRNAs are found even in viruses, in bacteria they should play an important regulatory role, even more so.
It turned out that prokaryotes have quite a few small RNA regulators. All of them can be conditionally divided into two groups:
- RNA molecules that must bind to proteins in order to perform their function.
- RNAs that complementarily bind to other RNAs (make up the majority of known regulatory RNA molecules).
In the first group, small RNAs are isolated, for which protein binding is possible, but not necessary. A well-known example is RNase P (RNAse P), which acts as a ribozyme on "maturing" tRNA. However, if RNase P can function without a protein component, then for other small RNAs in this group binding to the protein is necessary (and they themselves are, in fact, cofactors). For example, tmRNA activates a complex protein complex, acting as a "pick" for a "stuck" ribosome - if the messenger RNA from which the reading is carried out has come to an end and the stop codon has not been encountered.
An even more intriguing mechanism of direct interaction of small RNAs with proteins is also known. In any cell, proteins that bind to "traditional" nucleic acids are widespread. The prokaryotic cell is no exception. For example, its histone-like proteins help to correctly pack the DNA strand, and specific repressor proteins have an affinity for the operator region of bacterial genes. It has been shown that these repressors can be inhibited by small RNAs that mimic the DNA binding sites “native” for these proteins. Thus, on the small RNA CsrB (Fig. 1), there are 18 “bogus” sites that serve to prevent the repressor protein CsrA from reaching its true target, the glycogen operon. By the way, among the repressor proteins “lost” due to such small RNAs, there are regulators of global metabolic pathways, which makes it possible to multiply the inhibitory signal of small RNA. For example, this is done by small RNA 6S, which “mimics” the protein factor σ 70. By occupying the binding sites of the RNA polymerase with the sigma factor by a configuration "trick", it inhibits the expression of housekeeping genes.
Figure 1. Bioinformatically predicted secondary structure of small RNA CsrB from Vibrio cholerae M66-2. Small RNAs are single-stranded molecules, but, as with other RNAs, folding (folding) into a stable spatial structure is accompanied by the formation of regions where the molecule hybridizes to itself. Numerous bends on the structure in the form of open rings are called hairpins... In some cases, the combination of hairpins allows the RNA to act as a "sponge" by non-covalently binding certain proteins. But more often, molecules of this type interfere with DNA or RNA; in this case, the spatial structure of small RNA is disrupted, and new regions of hybridization are formed already with the target molecule. The heat map reflects the probability that the corresponding nucleotide pair will actually be bound by an intramolecular hydrogen bond; for unpaired sites, the probability of forming hydrogen bonds with any sites within the molecule. The image was obtained using the program RNAfold.
Small RNAs of bacteria interfere ... and very successfully!
The mechanism by which the regulators of the second group act, in general, is similar to that of regulatory RNAs of eukaryotes - this is the same RNA interference by hybridization with mRNA, only the small RNA chains themselves are often more authentic - up to several hundred nucleotides ( cm. fig. 1). As a result, because of the small RNA, the ribosomes cannot read information from the mRNA. Although it often does not seem to come to this: the formed complexes "small RNA - mRNA" become the target of RNases (such as RNase P).
The compactness and packing density of the prokaryotic genome makes itself felt: if in eukaryotes most of the regulatory RNAs are recorded in separate (most often not coding for a protein) loci, then many small RNAs of bacteria can be encoded in the same DNA region as the suppressed gene, but on the opposite chains! Such RNAs are called cis-encoded (antisense), and small RNAs lying at some distance from the inhibited DNA region - trans-coded... Apparently, the location of cis-RNA can be considered a triumph of ergonomics: they can be read from the opposite DNA strand at the moment of its unwinding simultaneously with the target transcript, which allows fine control of the amount of synthesized protein.
Small RNAs in the trans position evolve independently of the target mRNA, and the sequence of the regulator changes more as a result of mutations. It is possible that such an alignment of the bacterial cell is only "at hand", since small RNA acquires activity against previously uncharacteristic targets, which reduces the time and energy costs for creating other regulators. On the other hand, selection pressure prevents the trans-small RNA from mutating too much, as it will lose activity. Nevertheless, for hybridization with messenger RNA, most trans-small RNAs require an assistant, the Hfq protein. Apparently, otherwise incomplete complementarity of small RNA can create problems for binding to the target.
Apparently, a potentially realizable mechanism of regulation according to the principle of “one small RNA - many targets” helps to integrate the metabolic networks of bacteria, which is extremely necessary under conditions of a short single-celled life. We can continue to speculate on the topic and assume that with the help of trans-encoded small RNAs, the transfer of expression "prescriptions" from functionally related but physically distant loci is carried out. The need for this kind of genetic "roll call" logically explains the large number of small RNAs found in pathogenic bacteria. For example, several hundred small RNAs were found in the record holder for this indicator - Vibrio cholerae ( Vibrio cholerae). This is a microorganism that can survive in the surrounding aquatic environment (both fresh and salty), and on aquatic mollusks, and in fish, and in the human intestine - here you cannot do without complex adaptation with the help of regulatory molecules!
CRISPR on guard for bacterial health
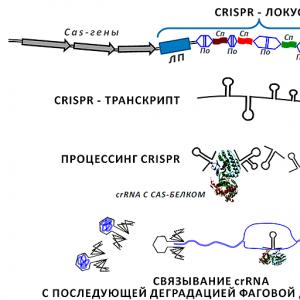
Small RNA was also used in solving another problem vital for bacteria. Even the most vicious pathogenic cocci and bacilli can be powerless in the face of the danger posed by special viruses - bacteriophages, capable of exterminating the bacterial population with lightning speed. Multicellular organisms have a specialized system to protect themselves from viruses - immune, by means of cells and substances secreted by them, protecting the body from intruders (including those of a viral nature). The bacterial cell is a loner, but it is not as vulnerable as it might seem at first glance. Loci act as keepers of recipes for maintaining the antiviral immunity of bacteria CRISPR - cluster regular-discontinuous short palindromic repeats ( clustered regularly interspaced short palindromic repeats) (Fig. 2;). In the genomes of prokaryotes, each CRISPR cassette is represented by a leader sequence several hundred nucleotides long, followed by a series of 2–24 (sometimes up to 400) repeats separated by spacer regions that are similar in length but unique in nucleotide sequence. The length of each spacer and repeat does not exceed one hundred base pairs.

Figure 2. CRISPR locus and processing of the corresponding small RNA to a functional transcript. In the genome CRISPR- the cassette is represented by interlaced spacers (in the figure they are designated as Cn), partially homologous to regions of phage DNA, and repeats ( By) 24–48 bp long, demonstrating dyadic symmetry. In contrast to repeats, spacers within one locus are the same in length (in different bacteria this can be 20–70 nucleotides), but differ in nucleotide sequence. The “-spacer-repeat-” sections can be quite long and consist of several hundred links. The entire structure is flanked on one side by the leader sequence ( LP, several hundred base pairs). Cas genes ( CRISPR-associated), organized into an operon. The proteins read from them perform a number of auxiliary functions, providing the processing of the transcript read from CRISPR-locus, its successful hybridization with the target phage DNA, insertion of new elements into the locus, etc. The CrRNA formed as a result of multistage processing hybridizes with a region of DNA (lower part of the figure) injected by the phage into the bacterium. This silences the virus transcription machine and stops its reproduction in the prokaryotic cell.
The detailed mechanism of the origin of everything CRISPR-locus remains to be studied. But to date, a schematic diagram of the emergence of spacers - the most important structures in its composition - has been proposed. It turns out that the "bacteria hunters" turn out to be beaten by their own weapon - nucleic acids, or rather, "trophy" genetic information received by bacteria from phages in previous battles! The point is that not all phages that enter a bacterial cell turn out to be fatal. The DNA of such phages (possibly related to temperate ones) is cut by special Cas proteins (their genes flank CRISPR) into small fragments. Some of these fragments will be embedded in CRISPR-loci of the "master" genome. And when phage DNA enters the bacterial cell again, it meets with small RNA from CRISPR-locus, at that moment expressed and processed by Cas-proteins. This is followed by inactivation of viral genetic information by the mechanism of RNA interference already described above.
From the hypothesis of the formation of spacers, it is not clear why repeats are needed between them, within one locus slightly differing in length, but almost identical in sequence? There is a wide scope for imagination here. Perhaps, without repetitions, it would be problematic to divide genetic data into semantic fragments, similar to sectors on a computer's hard disk, and then the access of the transcription machine to strictly defined areas CRISPR-locus would be embarrassing? Or maybe repeats simplify recombination processes when new elements of phage DNA are inserted? Or are they the "punctuation marks" that are indispensable in CRISPR processing? Be that as it may, the biological reason explaining the behavior of the bacterial cell in the manner of Gogol's Plyushkin will in due time be found.
CRISPRbeing a "chronicle" of the relationship between bacteria and phages, it can be used in phylogenetic studies. So, recently carried out typing by CRISPR allowed us to take a look at the evolution of individual strains of the plague microbe ( Yersinia pestis). Research their CRISPR- "pedigrees" shed light on the events of half a millennium ago, when the strains entered Mongolia from the territory now belonging to China. But not for all bacteria, and, in particular, pathogens, this method is applicable. Despite recent information on predicted CRISPR-processing proteins in tularemia pathogens ( Francisella tularensis) and cholera, CRISPRs themselves, if present in their genome, are not numerous. Perhaps, given their positive contribution to the acquisition of virulence by pathogenic members of the bacterial kingdom, phages are not so harmful and dangerous to defend against them using CRISPR? Or are the viruses attacking these bacteria too diverse, and the strategy of "interfering" RNA immunity against them is sterile?

Figure 3. Some mechanisms of riboswitch operation. Riboswitches (riboswitches) are built into messenger RNA, but differ in a large freedom of conformational behavior, depending on specific ligands, which gives reason to consider riboswitches as independent units of small RNAs. A change in the conformation of the expression platform affects the site of ribosome entry on mRNA ( RBS), and, as a consequence, determines the availability of all mRNA for reading. Riboswitches are to a certain extent similar to the operator domain in the classical model lac-operone - but only aptameric regions are usually regulated by low-molecular substances and switch the work of the gene at the level of mRNA, not DNA. and - In the absence of ligands, riboswitches btuB (cobalamin transporter) and thiM (thiamine pyrophosphate-dependent)that carry out non-nucleolytic repression of mRNA are "turned on" ( ON) and let the ribosome go about its business. Binding of the ligand to the riboswitch ( OFF-position) leads to the formation of a hairpin, making this site inaccessible to the ribosome. b - Lysine riboswitch lysC in the absence of a ligand is also included ( ON). Turning off the riboswitch blocks the ribosome's access to mRNA. But in contrast to the riboswitches described above, in the lysine, when turned off, the area is "exposed", cut by a special RNase complex ( degradosome), and all mRNA is utilized, breaking down into small fragments. Repression by riboswitch in this case is called nucleolytic ( nucleolytic) and is irreversible, since, in contrast to the example ( and ), switch back (again in ON) is no longer possible. It is important to note that utilization of a group of "unnecessary" mRNAs can be achieved in this way: the riboswitch is similar to a detail of a children's designer, and a whole group of functionally linked matrix molecules can have similar switches in structure.
Riboswitch - sensor for bacteria
So, there are protein-associated small RNAs, there are small RNAs that interfere with the bacteria's own mRNAs, as well as RNAs captured by bacteria from viruses and suppressing phage DNA. Is it possible to imagine any other mechanism of regulation with the help of small RNAs? It turns out, yes. If we analyze the above, it will be found that in all cases of antisense regulation there is interference between small RNA and the target as a result of hybridization of two individual molecules. Why not arrange small RNA as part of the transcript itself? Then, by changing the conformation of such a "sent Cossack" within the mRNA, it is possible to change the availability of the entire template for reading during translation, or, which is energetically even more expedient, to regulate the biosynthesis of mRNA, i.e. transcription!
Such structures are widely represented in bacterial cells and are known as riboswitches ( riboswitch). They are located before the beginning of the coding part of the gene, at the 5 'end of the mRNA. Conventionally, in the composition of riboswitches, two structural motives can be distinguished: aptamer regionresponsible for binding to the ligand (effector), and expression platformproviding regulation of gene expression through the transition of mRNA to alternative spatial structures. For example, such a switch ("off" type) is used to operate lysine operon: with an excess of lysine, it exists in the form of an “entangled” spatial structure that blocks reading from the operon, and with a lack of lysine, the riboswitch “unravels”, and proteins necessary for the biosynthesis of lysine are synthesized (Fig. 3).
The described schematic diagram of the riboswitch device is not a canon, there are options. An interesting "turning on" tandem riboswitch was found in Vibrio cholerae: the expression platform is preceded by two at once aptameric site. Obviously, this provides greater sensitivity and a smoother response to the appearance of another amino acid in the cell, glycine. Perhaps, it is indirectly involved in the high survival rate of the bacterium, which is similar in principle of action, but a "double" riboswitch in the genome of the causative agent of anthrax ( Bacillus anthracis). It reacts to thiamine pyrophosphate, which is vital for this microbe, which is part of the minimal environment.
In addition to switching metabolic pathways depending on the "menu" available to the bacterial cell, riboswitches can be sensors of bacterial homeostasis. Thus, they have been seen in the regulation of the gene's readability when the functioning of the translational system inside the cell is disrupted (for example, signals such as the appearance of "uncharged" tRNA and "faulty" (stalled) ribosomes), or when environmental factors change (for example, an increase in temperature ).
No protein needed, give us RNA!
So what does the presence of such a variety of small RNA regulators within bacteria mean? Does this indicate a rejection of the concept when the main "managers" are squirrels, or are we witnessing another fashion trend? Apparently, neither one nor the other. Certainly, some small RNAs are global regulators of metabolic pathways, like the aforementioned CsrB, which, together with CsrC, is involved in the regulation of organic carbon storage. But taking into account the principle of duplication of functions in biological systems, small RNAs of bacteria can be compared more with the "crisis manager" than with the CEO. So, in conditions when for the survival of a microorganism it is necessary fast reconfigure intracellular metabolism, their regulatory role may be decisive and more effective than proteins with similar functions. Thus, RNA regulators are more likely responsible for an express response, which is less stable and reliable than in the case of proteins: one should not forget that small RNA maintains its 3D structure and is retained on the inhibited matrix by weak hydrogen bonds.
The already mentioned small RNA of Vibrio cholerae can provide an indirect confirmation of these theses. For this bacterium, entering the human body is not a desirable goal, but, apparently, an emergency. The production of toxins and the activation of other, virulence-related pathways in this case is just a defensive reaction to the aggressive counteraction of the environment and body cells to "outsiders." “Rescuers” here are small RNAs - for example, Qrr, which help a vibrio in stressful conditions to modify its survival strategy by changing collective behavior. This hypothesis can also be indirectly confirmed by the discovery of a small RNA VrrA, which is actively synthesized when vibrios are found in the body and suppresses the production of Omp membrane proteins. “Hidden” membrane proteins in the initial phase of infection may help to avoid a powerful immune response from the human body (Fig. 4).

Figure 4. Small RNAs in the implementation of the pathogenic properties of Vibrio cholerae. and - Vibrio cholerae feels good and reproduces well in the aquatic environment. The human body is probably not the main ecological niche for this microbe. b - Getting through the water or food route of transmission of infection into an aggressive environment - the small intestine of a person - vibrios in terms of organized behavior begin to resemble a pseudoorganism, the main task of which is to restrain the immune response and create a favorable environment for colonization. Membrane vesicles are of great importance in the coordination of actions within the bacterial population and their interaction with the body. Until the end, unknown environmental factors in the intestine are signals for expression in vibrios of small RNAs (for example, VrrA). As a result, the mechanism of formation of vesicles is triggered, which are non-immunogenic with a low number of vibrio cells in the intestine. In addition to the described effect, small RNAs help to "hide" the membrane proteins Omp, potentially provocative for the human immune system. With the indirect participation of small RNAs Qrr1-4, an intensive production of cholera toxin is triggered (not shown in the figure), which complements the spectrum of adaptive responses of vibrio cholerae. in - Already after a few hours, the number of bacterial cells increases, and the pool of VrrA small RNAs decreases, which probably leads to the exposure of membrane proteins. The number of "empty" vesicles also gradually decreases, and at this stage they are replaced by immunogenic vesicles delivered to the enterocytes. Apparently, this is part of the "plan" for the implementation of a complex signal, the meaning of which is to provoke the evacuation of vibrios from the human body. NB: the ratio of the sizes of bacterial cells and enterocytes is not observed.
It is interesting to trace how our ideas about small RNA regulators will change when new data are obtained on the RNAseq platforms, including those on free-living and uncultured forms. Recent work using "deep sequencing" has already produced unexpected results, indicating the presence of microRNA-like molecules in mutant streptococci. Of course, such data need to be carefully checked, but, be that as it may, it is safe to say that the study of small RNAs in bacteria will present many surprises.
Acknowledgments
Original ideas and compositional design when creating the title picture, as well as picture 4, belong to E.A. Kopaeva, a graduate of the Iarch Institute of SFedU. The presence of figure 2 in the article is the merit of the associate professor of the department. Zoology SFedU G.B. Bakhtadze. He also carried out scientific proofreading and revision of the title figure and figure 4. The author expresses his deep gratitude to them for their patience and creative approach to the matter. Special thanks to my colleague, senior researcher lab. Biochemistry of Microbes of the Rostov Anti-Plague Institute V.M. Sorokin for the discussion of the text of the article and the expressed valuable comments.
Literature
- Karl Woese (1928–2012) ;;. 80 , 1148-1154;
- R. R. Breaker. (2012). Riboswitches and the RNA World. Cold Spring Harbor Perspectives in Biology. 4 , a003566-a003566;
- J. Patrick Bardill, Brian K. Hammer. (2012). Non-coding sRNAs regulate virulence in the bacterial pathogen Vibrio cholerae. RNA Biology. 9 , 392-401;
- Heon-Jin Lee, Su-Hyung Hong. (2012). Analysis of microRNA-size, small RNAs in Streptococcus mutans by deep sequencing. FEMS Microbiol Lett. 326 , 131-136;
- M.-P. Caron, L. Bastet, A. Lussier, M. Simoneau-Roy, E. Masse, D. A. Lafontaine. (2012). Dual-acting riboswitch control of translation initiation and mRNA decay. Proceedings of the National Academy of Sciences. 109 , E3444-E3453.
), preventing the translation of mRNA on ribosomes into the protein encoded by it. Ultimately, the effect of small interfering RNAs is identical to that if gene expression simply decreased.
Small interfering RNAs were discovered in 1999 by David Baulcombe's group in the UK as a component of the post-transcriptional gene silencing system in plants. PTGS, en: post-transcriptional gene silencing). The group published the findings in the journal Science.
Double-stranded RNAs can enhance gene expression by a mechanism called RNA-dependent gene activation (eng. RNAa, small RNA-induced gene activation). It has been shown that double-stranded RNAs complementary to the promoters of target genes cause the activation of the corresponding genes. RNA-dependent activation with the introduction of synthetic double-stranded RNAs has been shown in human cells. It is not known whether a similar system exists in the cells of other organisms.
Providing the ability to turn off essentially any gene at will, RNA interference based on small interfering RNAs has generated tremendous interest in basic and applied biology. The number of large-scale RNA interference tests to identify important genes in biochemical pathways is constantly growing. Since disease progression is also driven by gene activity, it is expected that, in some cases, gene shutdown by small interfering RNA may have a therapeutic effect.
However, the application of RNA interference based on small interfering RNAs to animals, and in particular to humans, faces many difficulties. Experiments have shown that the efficiency of small interfering RNAs is different for different types of cells: some cells easily respond to the effects of small interfering RNAs and demonstrate a decrease in gene expression, while in others this is not observed, despite effective transfection. The reasons for this phenomenon are still poorly understood.
The results of the first phase of trials of the first two therapeutic drugs acting on the RNA interference mechanism (intended for the treatment of macular degeneration), published at the end of 2005, show that drugs based on small interfering RNAs are easily tolerated by patients and have acceptable pharmacokinetic properties.
Preliminary clinical trials of small interfering RNAs targeting the Ebola virus indicate that they may be effective for post-exposure prophylaxis of the disease. This drug allowed the entire group of experimental primates who received a lethal dose of the Zaire Ebolavirus to survive
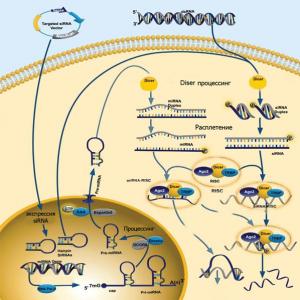
Destruction of the target mRNA can also occur under the influence of small interfering RNA (siRNA). RNA interference is one of the new revolutionary discoveries in molecular biology, and its authors received the Nobel Prize in 2002 for it. Interfering RNAs are very different in structure from other types of RNA and are two complementary RNA molecules approximately 21-28 nitrogenous bases long, which are connected to each other like strands in a DNA molecule. In this case, two unpaired nucleotides always remain at the edges of each of the siRNA strands. The impact is carried out as follows. When the siRNA molecule is inside the cell, it first binds to a complex with two intracellular enzymes - helicase and nuclease. This complex was named RISC ( RNA- induced silencing complex; silence - eng. be silent, shut up; silencing - silence, as the process of "turning off" a gene is called in the English language and special literature). Next, the helicase untwists and separates the siRNA strands, and one of the strands (antisense in structure) in a complex with the nuclease specifically interacts with the complementary (strictly corresponding) region of the target mRNA, which allows the nuclease to cut it into two parts. The cut sections of the mRNA are further exposed to the action of other cellular RNA nucleases, which further cut them into smaller pieces.
Found in plants and lower animal organisms (insects) siRNAs are an important link in a kind of "intracellular immunity" that allows you to recognize and quickly destroy foreign RNA. In the event that an RNA-containing virus penetrates the cell, such a defense system will prevent it from multiplying. If the virus contains DNA, the siRNA system will prevent it from producing viral proteins (since the mRNA required for this will be recognized and cut), and using this strategy will slow down its spread throughout the body. The siRNA system was found to be extremely legible: each siRNA will recognize and destroy only its own specific mRNA. Substitution of only one nucleotide within the siRNA leads to a dramatic decrease in the effect of interference. None of the gene blockers known so far has this exceptional specificity for its target gene.
Currently, this method is used mainly in scientific research to identify the functions of various cellular proteins. However, it can potentially also be used to create medicines.
The discovery of RNA interference has given new hope in the fight against AIDS and cancer. Perhaps, by using siRNA therapy in conjunction with traditional antiviral and anticancer therapies, it is possible to achieve a potentiation effect, where the two effects lead to a more pronounced therapeutic effect than the simple sum of each of them applied separately.
In order to use the siRNA interference mechanism in mammalian cells for therapeutic purposes, ready-made double-stranded siRNA molecules must be introduced into the cells. However, there are a number of problems that currently do not allow for this in practice, and even more so to create some kind of dosage forms. Firstly, the first echelon of the body's defense acts on them in the blood, enzymes - nucleasesthat cut potentially dangerous and unusual for our body double RNA strands. Secondly, despite their name, small RNAs are still quite long, and, most importantly, they carry a negative electrostatic charge, which makes their passive penetration into the cell impossible. And thirdly, one of the most important questions is how to make siRNA work (or penetrate) only in certain ("sick") cells, without affecting healthy ones? And finally, the problem of size. The optimum size for such synthetic siRNAs is the same 21-28 nucleotides. If you increase its length, cells will respond by producing interferon and reducing protein synthesis. On the other hand, if one tries to use siRNAs smaller than 21 nucleotides, the specificity of its binding to the desired mRNA and the ability to form an RISC complex are sharply reduced. It should be noted that overcoming these problems is critical not only for siRNA therapy, but for gene therapy in general.
Some progress has already been made in their solution. For example, scientists are trying to make siRNA molecules more lipophilic, that is, they can dissolve in the fats that make up the cell membrane, and thus facilitate the penetration of siRNA into the cell. And in order to ensure the specificity of work within only certain tissues, genetic engineers include in their structures special regulatory regions that are activated and trigger the reading of information contained in a similar structure (and therefore siRNA, if it is included there), only in certain cells fabrics.
For example, researchers from the San Diego School of Medicine at the University of California (University of California, San Diego School of Medicine) have developed a new efficient delivery system for small interfering RNAs (siRNAs), which suppress the production of certain proteins, into cells. This system should become the basis for a technology for the specific delivery of drugs to various types of cancer. “Small interfering RNAs, which carry out a process called RNA interference, have incredible potential for cancer treatment,” explains Professor Steven Dowdy, who led the study, “and while we still have a lot to do, we have developed the technology delivery of drugs to the population of cells - both primary tumor and metastases, without damaging healthy cells ”.
For many years, Dowdy and his colleagues have studied the anti-cancer potential of small interfering RNAs. However, ordinary siRNAs are tiny, negatively charged molecules that, due to their properties, are extremely difficult to deliver into the cell. To achieve this, the scientists used a short signaling protein PTD (peptide transduction domain). Previously, more than 50 “fusion proteins” were created with its use, in which PTD was combined with tumor suppressor proteins.
However, the simple combination of siRNA with PTD does not lead to the delivery of RNA into the cell: siRNAs are negatively charged, PTDs are positively charged, as a result of which a dense RNA-protein conglomerate is formed that is not transported across the cell membrane. Therefore, the researchers first linked PTD to a protein RNA-binding domain that neutralized the negative charge of siRNA (resulting in a fusion protein called PTD-DRBD). Such an RNA-protein complex already easily passes through the cell membrane and enters the cytoplasm of the cell, where it specifically inhibits the messenger RNAs of proteins that activate tumor growth.
To determine the ability of the PTD-DRBD fusion protein to deliver siRNA into cells, the scientists used a cell line derived from human lung cancer. After treatment of cells with PTD-DRBD-siRNA, it was found that tumor cells are most susceptible to siRNA, while in normal cells (T cells, endothelial cells and embryonic stem cells were used as controls), where there was no increased production of oncogenic proteins, no toxic effects were observed.
This method can be subjected to various modifications, using different siRNAs to suppress different tumor proteins - not only overproduced, but also mutant. It is also possible to modify the therapy in the case of recurrent tumors, which usually become resistant to chemotherapy drugs due to new mutations.
Oncological diseases are highly variable, and the molecular characteristics of tumor cell proteins are individual for each patient. The authors of the work believe that in this situation, the use of small interfering RNAs is the most rational approach to therapy.
Scientists believe that the incorrect expression of small RNAs is one of the causes of a number of diseases that very seriously affect the health of many people around the world. Among such diseases are cardiovascular 23 and oncological 24. As for the latter, this is not surprising: cancer testifies to abnormalities in the development of cells and in their fate, and small RNAs play an important role in the corresponding processes. Here is one of the most revealing examples of the enormous effect that small RNAs have on the body in cancer. We are talking about a malignant tumor, which is characterized by the incorrect expression of those genes that act during the initial development of the organism, and not in the postnatal period. It is a type of childhood brain tumor that usually appears before the age of two. Alas, this is a very aggressive form of cancer, and the prognosis is poor even with intensive treatment. The oncological process develops as a result of improper redistribution of genetic material in brain cells. The promoter, which usually causes strong expression of one of the genes encoding proteins, undergoes recombination with a specific small RNA cluster. Then this entire rearranged region is amplified: in other words, many copies of it are created in the genome. Consequently, small RNAs located “downstream” than the displaced promoter are expressed much more strongly than they should. The content of active small RNAs is approximately 150-1000 times higher than normal.
Fig. 18.3. Small RNAs activated by alcohol can bind to messenger RNAs that do not affect the body's resistance to alcohol. But these small RNAs do not bind to messenger RNA molecules that contribute to this resistance. This leads to a relative predominance of the proportion of messenger RNA molecules encoding protein variations associated with alcohol resistance.
This cluster encodes over 40 different small RNAs. Actually, this is generally the largest of such clusters available in primates. It is usually expressed only at an early stage of human development, in the first 8 weeks of an embryo's life. Its strong activation in the infant's brain leads to a catastrophic effect on genetic expression. One of the consequences is the expression of an epigenetic protein that adds modifications to the DNA. This leads to large-scale changes in the entire pattern of DNA methylation, and hence to abnormal expression of all kinds of genes, many of which must be expressed only when immature brain cells divide during the early stages of the body's development. This is how the cancer program 25 starts in the baby's cells.
Such communication between small RNAs and the epigenetic apparatus of a cell can have a significant impact on other situations when a predisposition to cancer develops in cells. This mechanism probably leads to the fact that the effect of impaired expression of small RNAs is enhanced by altering epigenetic modifications that are transmitted to daughter cells from the mother. This is how a scheme of potentially dangerous changes in the nature of gene expression can be formed.
So far, scientists have not figured out all the stages of the interaction of small RNAs with epigenetic processes, but they still manage to get some hints of what is happening. For example, it turned out that a certain class of small RNAs that increase the aggressiveness of breast cancer targets certain enzymes in messenger RNAs that remove key epigenetic modifications. This alters the pattern of epigenetic modifications in the cancer cell and further disrupts genetic expression 26.
Many forms of cancer are difficult to track in a patient. Oncological processes can take place in hard-to-reach places, which complicates the sampling procedure. In such cases, it is difficult for the doctor to monitor the development of the cancer process and the response to treatment. Often, physicians are forced to rely on indirect measurements - say, a tomographic scan of a tumor. Some researchers believe that small RNA molecules could help create a new method for monitoring tumor development, which also allows studying its origin. When cancer cells die, small RNAs leave the cells when they rupture. These small junk molecules often form complexes with cellular proteins or are wrapped in fragments of cell membranes. Due to this, they are very stable in body fluids, which means that such RNA can be isolated and analyzed. Since their numbers are small, researchers will have to use highly sensitive analytical methods. However, nothing is impossible here: the sensitivity of nucleic acid sequencing is constantly increasing 27. Published data confirming the promise of this approach in relation to breast cancer 28, ovarian cancer 29 and a number of other cancers. Analysis of small circulating RNAs in lung cancer patients has shown that these RNAs help distinguish between patients with a single pulmonary nodule (not requiring therapy) and patients who develop malignant tumor nodules (requiring treatment) 30.