How much heat is required for m grams. Quantity of heat
Mankind knows few types of energy - mechanical energy (kinetic and potential), internal energy (thermal), field energy (gravitational, electromagnetic and nuclear), chemical. Separately, it is worth highlighting the energy of the explosion, ...
The energy of the vacuum and still existing only in theory - dark energy. In this article, the first in the heading "Heat engineering", I will try in a simple and accessible language, using a practical example, to talk about the most important form of energy in people's lives - about thermal energy and about giving birth to her in time heat capacity.
A few words to understand the place of heat engineering as a branch of the science of obtaining, transferring and using thermal energy. Modern heat engineering has emerged from general thermodynamics, which in turn is one of the branches of physics. Thermodynamics is literally "warm" plus "power". Thus, thermodynamics is the science of "changing the temperature" of a system.
The impact on the system from the outside, in which its internal energy changes, may be the result of heat transfer. Thermal energy, which is acquired or lost by the system as a result of such interaction with the environment, is called the amount of warmth and is measured in SI units in Joules.
If you are not a heating engineer, and you do not deal with heat engineering issues every day, then when faced with them, sometimes without experience it is very difficult to quickly understand them. It is difficult, without experience, to imagine even the dimensionality of the sought values \u200b\u200bof the amount of heat and thermal power. How many Joules of energy is needed to heat 1000 cubic meters of air from a temperature of -37˚C to + 18 ..C? .. What is the power of a heat source to do this in 1 hour? .. These not the most difficult questions can be answered right away "Not all engineers. Sometimes specialists even remember the formulas, but only a few can apply them in practice!
After reading this article to the end, you can easily solve real industrial and domestic problems associated with heating and cooling various materials. Understanding the physical essence of heat transfer processes and knowing simple basic formulas are the main building blocks in the foundation of knowledge in heat engineering!
The amount of heat in various physical processes.
Most of the known substances can be in solid, liquid, gaseous or plasma states at different temperatures and pressures. Transition from one state of aggregation to another occurs at constant temperature (provided that the pressure and other environmental parameters do not change) and is accompanied by the absorption or release of thermal energy. Despite the fact that 99% of matter in the Universe is in a plasma state, we will not consider this state of aggregation in this article.
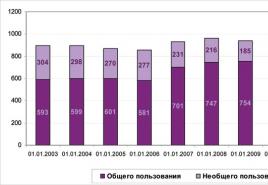
Consider the graph shown in the figure. It shows the dependence of the temperature of the substance T on the amount of heat Q , brought to a certain closed system containing a certain mass of a specific substance.

1. Solid body with temperature T1 , heat up to temperature Tm , spending on this process an amount of heat equal to Q1 .
2. Next, the melting process begins, which occurs at a constant temperature TPL (melting point). To melt the entire mass of a solid, it is necessary to expend heat energy in an amount Q2 - Q1 .
3. Next, the liquid resulting from the melting of a solid is heated to the boiling point (gas formation) Tkp , spending on this amount of heat equal to Q3-Q2 .
4. Now at constant boiling point Tkp the liquid boils and evaporates, turning into gas. To convert the entire mass of liquid into gas, it is necessary to expend thermal energy in the amount Q4-Q3.
5. At the last stage, the gas is heated from temperature Tkp to a certain temperature T2 ... In this case, the cost of the amount of heat will be Q5-Q4 ... (If we heat the gas to the ionization temperature, then the gas turns into plasma.)
Thus, heating the original solid from temperature T1 to temperature T2 we have spent heat energy in the amount Q5 , transferring the substance through three states of aggregation.
Moving in the opposite direction, we will remove the same amount of heat from the substance. Q5, passing through the stages of condensation, crystallization and cooling from temperature T2 to temperature T1 ... Of course, we are considering a closed system without energy loss to the external environment.
Note that a transition from a solid state to a gaseous state is possible, bypassing the liquid phase. Such a process is called sublimation, and the reverse process is called desublimation.
So, they realized that the processes of transitions between the states of aggregation of matter are characterized by the consumption of energy at a constant temperature. When a substance is heated in one constant state of aggregation, the temperature rises and thermal energy is also consumed.
The main formulas for heat transfer.
The formulas are very simple.
Quantity of heat Q in J is calculated by the formulas:
1. From the side of heat consumption, that is, from the load side:
1.1. When heating (cooling):
Q = m * c * (T2-T1)
m – mass of substance in kg
from -specific heat of a substance in J / (kg * K)
1.2. When melting (freezing):
Q = m * λ
λ – specific heat of fusion and crystallization of a substance in J / kg
1.3. Boiling, evaporation (condensation):
Q = m * r
r – specific heat of gas formation and condensation of a substance in J / kg
2. From the heat production side, that is, from the source side:
2.1. During fuel combustion:
Q = m * q
q – specific heat of combustion of fuel in J / kg
2.2. When converting electricity into thermal energy (Joule-Lenz law):
Q \u003d t * I * U \u003d t * R * I ^ 2 \u003d (t / R)* U ^ 2
t – time in s
I – effective current in A
U – effective voltage value in V
R – load resistance in ohms
We conclude that the amount of heat is directly proportional to the mass of the substance during all phase transformations and, when heated, is additionally directly proportional to the temperature difference. The proportionality coefficients ( c , λ , r , q ) for each substance have their own values \u200b\u200band are determined empirically (taken from reference books).
Thermal power N in W is the amount of heat transferred to the system for a certain time:
N \u003d Q / t
The faster we want to heat the body to a certain temperature, the more power the source of thermal energy should be - everything is logical.
Calculation in Excel of an applied problem.
In life, it is often necessary to make a quick estimate calculation in order to understand whether it makes sense to continue studying the topic, making a project and detailed accurate labor-intensive calculations. Having made a calculation in a few minutes, even with an accuracy of ± 30%, you can make an important management decision that will be 100 times cheaper and 1000 times more operational and, as a result, 100,000 times more efficient than performing an accurate calculation within a week, otherwise and a month, by a group of expensive specialists ...
Conditions of the problem:
In the premises of the workshop for the preparation of rolled metal with dimensions of 24m x 15m x 7m, we import metal products in the amount of 3 tons from a warehouse on the street. The rolled metal has ice with a total weight of 20 kg. On the street -37˚С. What amount of heat is needed to heat the metal to + 18˚С; heat the ice, melt it and heat the water to + 18˚С; heat the entire volume of air in the room, assuming that the heating was completely turned off before? What capacity should the heating system have if all of the above must be done in 1 hour? (Very harsh and almost unrealistic conditions - especially when it comes to air!)
We will perform the calculation in the programMS Excel or in the programOOo Calc.
For color formatting of cells and fonts, see the page "".
Initial data:
1. We write the names of the substances:
to cell D3: Steel
to cell E3: Ice
into cell F3: Ice / water
to cell G3: Water
to cell G3: Air
2. We enter the names of the processes:
into cells D4, E4, G4, G4: heat
into cell F4: melting
3. Specific heat of substances c in J / (kg * K) we write for steel, ice, water and air, respectively
to cell D5: 460
to cell E5: 2110
to cell G5: 4190
to cell H5: 1005
4. Specific heat of melting of ice λ in J / kg we enter
into cell F6: 330000
5. Mass of substances m in kg we enter, respectively, for steel and ice
to cell D7: 3000
to cell E7: 20
Since the mass does not change when ice turns into water, then
in cells F7 and G7: \u003d E7 =20
We find the mass of air by the product of the volume of the room by the specific gravity
in cell H7: \u003d 24 * 15 * 7 * 1.23 =3100
6. Process time t in min we write only once for steel
to cell D8: 60
The times for heating ice, melting it and heating the resulting water are calculated from the condition that all these three processes must be completed in the same amount of time as is allotted for heating the metal. We read accordingly
in cell E8: \u003d E12 / (($ E $ 12 + $ F $ 12 + $ G $ 12) / D8) =9,7
in cell F8: \u003d F12 / (($ E $ 12 + $ F $ 12 + $ G $ 12) / D8) =41,0
in cell G8: \u003d G12 / (($ E $ 12 + $ F $ 12 + $ G $ 12) / D8) =9,4
The air must also warm up during the same allotted time, read
in cell H8: \u003d D8 =60,0
7. The initial temperature of all substances T1 in ˚C we enter
to cell D9: -37
to cell E9: -37
to cell F9: 0
to cell G9: 0
to cell H9: -37
8. The final temperature of all substances T2 in ˚C we enter
to cell D10: 18
to cell E10: 0
to cell F10: 0
to cell G10: 18
to cell H10: 18
I think there should be no questions on clauses 7 and 8.

Calculation results:
9. Quantity of heat Q in KJ required for each of the processes we calculate
for heating steel in cell D12: \u003d D7 * D5 * (D10-D9) / 1000 =75900
for heating ice in compartment E12: \u003d E7 * E5 * (E10-E9) / 1000 = 1561
to melt ice in cell F12: \u003d F7 * F6 / 1000 = 6600
for heating water in cell G12: \u003d G7 * G5 * (G10-G9) / 1000 = 1508
for heating air in cell H12: \u003d H7 * H5 * (H10-H9) / 1000 = 171330
The total amount of heat energy required for all processes is read
in merged cell D13E13F13G13H13: \u003d SUM (D12: H12) = 256900
In cells D14, E14, F14, G14, H14, and in the combined cell D15E15F15G15H15, the amount of heat is given in the arc unit of measurement - in Gcal (in giga calories).
10. Thermal power N in kW, required for each of the processes is calculated
for heating steel in cell D16: \u003d D12 / (D8 * 60) =21,083
for heating ice in cell E16: \u003d E12 / (E8 * 60) = 2,686
to melt ice in cell F16: \u003d F12 / (F8 * 60) = 2,686
for heating water in cell G16: \u003d G12 / (G8 * 60) = 2,686
for heating air in cell H16: \u003d H12 / (H8 * 60) = 47,592
The total thermal power required to complete all processes in time t calculated
in merged cell D17E17F17G17H17: \u003d D13 / (D8 * 60) = 71,361
In cells D18, E18, F18, G18, H18, and in the combined cell D19E19F19G19H19, the thermal power is given in the arc unit of measurement - in Gcal / hour.
This completes the calculation in Excel.
Conclusions:
Please note that heating the air requires more than twice as much energy as heating the same mass of steel.
When heating water, energy consumption is twice as much as when heating ice. The melting process consumes many times more energy than the heating process (with a small temperature difference).
Heating water consumes ten times more heat energy than heating steel and four times more than heating air.
For receiving information about the release of new articles and for download of working program files i ask you to subscribe to announcements in the window located at the end of the article or in the window at the top of the page.
After entering your email address and clicking on the "Receive article announcements" DO NOT FORGETCONFIRM SUBSCRIBE by clicking on the link in a letter that will immediately come to you to the specified mail (sometimes - to the folder « Spam » )!
We remembered the concepts of "amount of heat" and "thermal power", considered the fundamental formulas of heat transfer, and analyzed a practical example. I hope my language was simple, clear and interesting.
I am waiting for questions and comments on the article!
I beg RESPECT author's work download file AFTER SUBSCRIPTION for article announcements.
“… - How many parrots can fit in you, that is your height.
- Really needed! I will not swallow so many parrots! ... ”
From m / f "38 parrots"
In accordance with international rules SI (international system of units of measurement), the amount of heat energy or the amount of heat is measured in Joules [J], there are also multiples of kiloJoule [kJ] \u003d 1000 J., MegaJoule [MJ] \u003d 1,000,000 J, GigaJoule [ GJ] \u003d 1,000,000,000 J., etc. This unit of measurement of thermal energy is the main international unit and is most often used in scientific and scientific-technical calculations.
However, all of us know, or at least once have heard, and another unit for measuring the amount of heat (or just heat) is calorie, as well as kilocalorie, Megacalorie and Gigacalorie, which mean the prefixes kilo, Giga and Mega, see the example with Joules above. In our country, historically, when calculating tariffs for heating, whether it is heating with electricity, gas or pellet boilers, it is customary to consider the cost of exactly one Gigacalorie of thermal energy.
So what is Gigacalorie, kiloWatt, kiloWatt * hour or kiloWatt / hour and Joules and how are they related ?, you will find out in this article.
So, the main unit of thermal energy is, as already mentioned, Joule. But before talking about the units of measurement, it is necessary, in principle, at the household level to explain what heat energy is and how and why it is measured.
We all know from childhood that in order to get warm (get heat energy) we need to set fire to something, so we all burned fires, the traditional fuel for a fire is wood. Thus, obviously, when burning fuel (any: firewood, coal, pellets, natural gas, diesel fuel), thermal energy (heat) is released. But in order to heat, for example, different volumes of water, a different amount of wood (or other fuel) is required. It is clear that to heat two liters of water, a few firewoods are enough, and to prepare half a bucket of soup for the whole camp, you need to stock up on several bundles of firewood. In order not to measure such strict technical quantities as the amount of heat and the heat of combustion of fuel with bundles of firewood and buckets of soup, heating engineers decided to clarify and order and agreed to invent a unit of the amount of heat. For this unit to be the same everywhere, it was defined as follows: to heat one kilogram of water by one degree under normal conditions (atmospheric pressure), 4,190 calories are required, or 4.19 kilocalories, therefore, to heat one gram of water, a thousand times less heat will be enough - 4.19 calories.
A calorie is related to the international unit of thermal energy - Joule by the following relationship:
1 calorie \u003d 4.19 Joule.
Thus, to heat 1 gram of water by one degree, 4.19 Joules of thermal energy are required, and to heat one kilogram of water, 4,190 Joules of heat.
In technology, along with the unit of measurement of thermal (and any other) energy, there is a unit of power and, in accordance with the international system (SI), it is Watt. The concept of power also applies to heating appliances. If a heating device is capable of giving 1 Joule of thermal energy in 1 second, then its power is 1 Watt. Power is the ability of a device to produce (create) a certain amount of energy (in our case, heat energy) per unit of time. Returning to our example with water, in order to heat one kilogram (or one liter, in the case of water, a kilogram is a liter) of water per one degree Celsius (or Kelvin, no difference), we need a power of 1 kilocalorie or 4,190 J. of thermal energy. To heat one kilogram of water in 1 second of time by 1 degree of degrees centigrade, we need a device of the following power:
4190 J / 1 s. \u003d 4 190 W. or 4.19 kW.
If we want to heat our kilogram of water by 25 degrees in the same second, then we need a power twenty-five times more, i.e.
4.19 * 25 \u003d 104.75 kW.
Thus, we can conclude that a pellet boiler with a capacity of 104.75 kW. heats 1 liter of water by 25 degrees in one second.
Since we got to Watts and kiloWatts, we should put in a word about them too. As already mentioned, Watt is a unit of power, including the thermal power of a boiler, but in addition to pellet boilers and gas boilers, mankind is also familiar with electric boilers, the power of which is measured, of course, in the same kiloWatts and they do not consume pellets or gas, and electricity, the amount of which is measured in kilowatt hours. The correct spelling of the unit of energy kiloWatt * hour (namely, kiloWatt is multiplied by an hour, not divided), writing kW / hour is a mistake!
In electric boilers, electric energy is converted into thermal energy (the so-called Joule heat), and if the boiler consumed 1 kW * hour of electricity, then how much heat did it generate? To answer this simple question, you need to do a simple calculation.
Convert kiloWatts to kiloJoules / seconds (kiloJoules per second), and hours to seconds: in one hour, 3600 seconds, we get:
1 kW * hour \u003d [1 kJ / s] * 3600 s. \u003d 1,000 J * 3600 s \u003d 3,600,000 Joules or 3.6 MJ.
So,
1 kW * hour \u003d 3.6 MJ.
In turn, 3.6 MJ / 4.19 \u003d 0.859 Mcal \u003d 859 kcal \u003d 859,000 cal. Energy (thermal).
Now let's move on to Gigacaloria, the price of which on various types of fuel is used by heating engineers.
1 Gcal \u003d 1,000,000,000 cal.
1,000,000,000 cal. \u003d 4.19 * 1,000,000,000 \u003d 4,190,000,000 J \u003d 4,190 MJ. \u003d 4.19 GJ.
Or knowing that 1 kW * hour \u003d 3.6 MJ, we recalculate 1 Gigacaloria per kilowatt * hour:
1 Gcal \u003d 4190 MJ / 3.6 MJ \u003d 1 163 kW * hours!
If, after reading this article, you decide to consult a specialist of our company on any issue related to heat supply, then you Here!
Source: teplo-en.ru
730. Why is water used to cool some mechanisms?
Water has a high specific heat, which contributes to good heat dissipation from the mechanism.
731. In which case it is necessary to spend more energy: for heating one liter of water by 1 ° C or for heating one hundred grams of water by 1 ° C?
To heat a liter of water, since the greater the mass, the more energy you need to spend.
732. Cupronickel and silver forks of the same mass were dipped into hot water. Will they receive the same amount of heat in water?
Cupronickel plug will receive more heat, because the specific heat of cupronickel is greater than that of silver.
733. A piece of lead and a piece of cast iron of the same mass were hit three times with a sledgehammer. Which piece is hotter?
Lead will heat up more because it has a lower specific heat than cast iron and requires less energy to heat the lead.
734. In one flask there is water, in the other there is kerosene of the same mass and temperature. An equally heated iron cube was thrown into each flask. Which heats up to a higher temperature - water or kerosene?
Kerosene.
735. Why in cities on the seashore temperature fluctuations in winter and summer are less sharp than in cities located in the interior of the continent?
Water heats up and cools more slowly than air. In winter, it cools down and moves warm air masses to land, making the climate on the coast warmer.
736. The specific heat capacity of aluminum is 920 J / kg ° C. What does this mean?
This means that to heat 1 kg of aluminum at 1 ° C, 920 J.
737. Aluminum and copper bars of the same weight 1 kg are cooled by 1 ° C. How much will the internal energy of each bar change? Which bar will it change more and by how much?
738. What amount of heat is needed to heat a kilogram iron billet by 45 ° C?
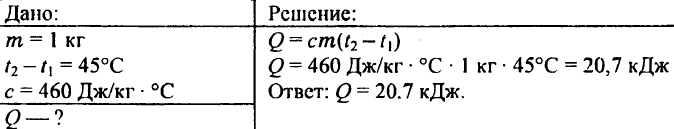
739. What amount of heat is required to heat 0.25 kg of water from 30 ° C to 50 ° C?

740. How will the internal energy of two liters of water change when heated by 5 ° C?

741. What amount of heat is needed to heat 5 g of water from 20 ° C to 30 ° C?

742. What amount of heat is needed to heat an aluminum ball weighing 0.03 kg at 72 ° C?

743. Calculate the amount of heat required to heat 15 kg of copper at 80 ° C.
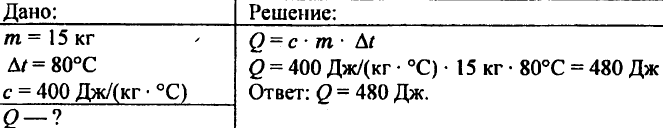
744. Calculate the amount of heat required to heat 5 kg of copper from 10 ° C to 200 ° C.
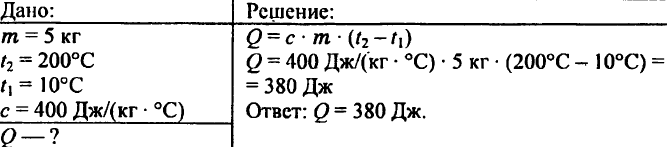
745. What amount of heat is required to heat 0.2 kg of water from 15 ° C to 20 ° C?

746. Water weighing 0.3 kg cooled down by 20 ° C. How much has the internal energy of water decreased?

747. What amount of heat is needed to heat 0.4 kg of water at a temperature of 20 ° C to a temperature of 30 ° C?

748. What amount of heat is spent on heating 2.5 kg of water at 20 ° C?
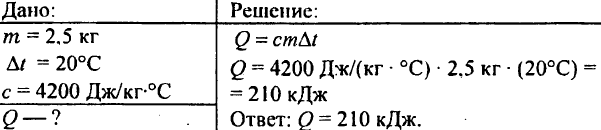
749. What amount of heat was released during cooling of 250 g of water from 90 ° C to 40 ° C?

750. What amount of heat is required to heat 0.015 liters of water by 1 ° C?
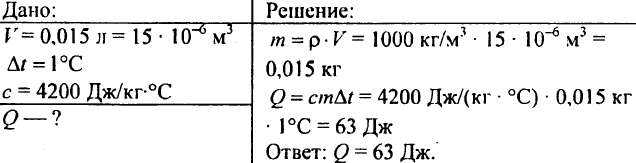
751. Calculate the amount of heat required to heat a 300 m3 pond by 10 ° C?
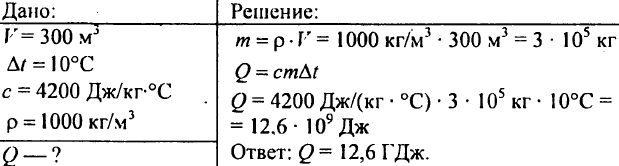
752. What amount of heat must be imparted to 1 kg of water in order to raise its temperature from 30 ° С to 40 ° С?

753. Water with a volume of 10 liters has cooled from a temperature of 100 ° C to a temperature of 40 ° C. How much heat was released during this?

754. Calculate the amount of heat required to heat 1 m3 of sand to 60 ° C.

755. Air volume 60 m3, specific heat 1000 J / kg ° С, air density 1.29 kg / m3. How much heat is needed to heat it to 22 ° C?

756. The water was heated by 10 ° C, using 4.20 103 J of heat. Determine the amount of water.
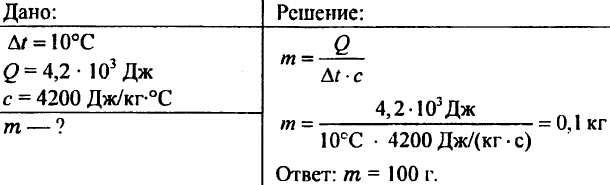
757. Water weighing 0.5 kg was reported 20.95 kJ of heat. What was the water temperature if the initial water temperature was 20 ° C?

758. A copper saucepan weighing 2.5 kg is filled with 8 kg of water at 10 ° C. How much heat is needed to bring the water in a saucepan to a boil?

759. A liter of water at a temperature of 15 ° C is poured into a copper ladle weighing 300 g. What amount of heat is needed to heat water in a ladle to 85 ° C?

760. A piece of heated granite weighing 3 kg is placed in water. Granite transfers 12.6 kJ of heat to water, cooling by 10 ° C. What is the specific heat of the stone?
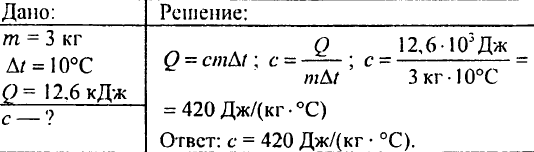
761. Hot water at 50 ° C was added to 5 kg of water at 12 ° C to obtain a mixture with a temperature of 30 ° C. How much water was added?

762. Water at 20 ° C was added to 3 liters of water at 60 ° C to obtain water at 40 ° C. How much water was added?

763. What will be the temperature of the mixture if you mix 600 g of water at 80 ° C with 200 g of water at 20 ° C?

764. A liter of water at 90 ° C was poured into water at 10 ° C, and the temperature of the water became 60 ° C. How much cold water was there?

765. Determine how much hot water heated to 60 ° C should be poured into the vessel if the vessel already contains 20 liters of cold water at a temperature of 15 ° C; the temperature of the mixture should be 40 ° C.

766. Determine how much heat is required to heat 425 g of water at 20 ° C.

767. How many degrees will 5 kg of water heat up if the water receives 167.2 kJ?

768. How much heat is required to heat m grams of water at temperature t1 to temperature t2?
769. The calorimeter is filled with 2 kg of water at a temperature of 15 ° C. To what temperature will the calorimeter water be heated if a brass weight of 500 g, heated to 100 ° C, is lowered into it? The specific heat capacity of brass is 0.37 kJ / (kg ° C).

770. There are lumps of copper, tin and aluminum of the same volume. Which of these pieces has the highest and lowest heat capacity?

771. The calorimeter was filled with 450 g of water, the temperature of which was 20 ° C. When 200 g of iron filings heated to 100 ° C were immersed in this water, the water temperature became 24 ° C. Determine the specific heat of the sawdust.
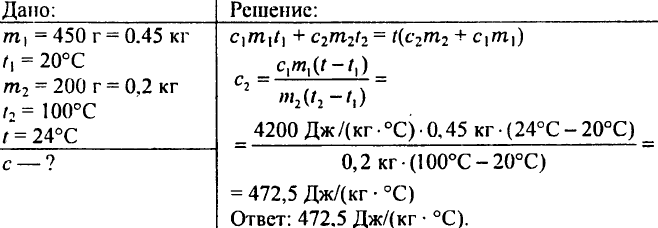
772. A copper calorimeter weighing 100 g contains 738 g of water, the temperature of which is 15 ° C. 200 g of copper was dropped into this calorimeter at a temperature of 100 ° C, after which the temperature of the calorimeter rose to 17 ° C. What is the specific heat of copper?

773. A steel ball weighing 10 g is taken out of the oven and immersed in water with a temperature of 10 ° C. The water temperature rose to 25 ° C. What was the temperature of the ball in the oven if the mass of water was 50 g? The specific heat capacity of steel is 0.5 kJ / (kg ° C).
776. Water weighing 0.95 g at a temperature of 80 ° C was mixed with water weighing 0.15 g at a temperature of 15 ° C. Determine the temperature of the mixture. 779. A steel cutter weighing 2 kg was heated to a temperature of 800 ° C and then lowered into a vessel containing 15 liters of water at a temperature of 10 ° C. To what temperature will the water in the vessel be heated?

(Note. To solve this problem, it is necessary to compose an equation in which the unknown temperature of the water in the vessel after lowering the cutter is taken as the unknown.)
780. What is the temperature of water if you mix 0.02 kg of water at 15 ° C, 0.03 kg of water at 25 ° C and 0.01 kg of water at 60 ° C?

781. Heating of a well-ventilated class requires an amount of heat 4.19 MJ per hour. Water enters the heating radiators at 80 ° C, and leaves them at 72 ° C. How much water do you need to supply every hour to the radiators?

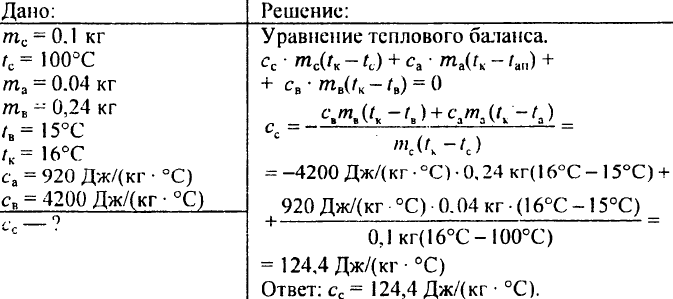
782. Lead weighing 0.1 kg at a temperature of 100 ° C was immersed in an aluminum calorimeter weighing 0.04 kg containing 0.24 kg of water at a temperature of 15 ° C. After that, the temperature in the calorimeter was set at 16 ° C. What is the specific heat of lead?
By definition, a calorie is the amount of heat required to heat one cubic centimeter of water by 1 degree Celsius. A gigacalorie, used to measure heat energy in thermal power engineering and utilities, is a billion calories. In 1 meter there are 100 centimeters, therefore, in one cubic meter - 100 x 100 x 100 \u003d 1,000,000 centimeters. Thus, to heat a cube of water by
1 degree, you need a million calories or 0.001 Gcal.
In my city, the heating price is 1132.22 rubles / Gcal, and the price of hot water is 71.65 rubles / cubic meter, the price of cold water is 16.77 rubles / cubic meter.
How much Gcal is spent to heat 1 cubic meter of water?
I think so
s x 1132.22 \u003d 71.65 - 16.77 and thus I solve the equations in order to find out what s (Gcal) is equal to, that is, equal to 0.0484711452 Gcal
I doubt it, in my opinion, I decide wrong
ANSWER:
I do not find errors in your calculation.
Naturally, the given tariffs should not include the cost of wastewater (wastewater disposal).
An approximate calculation for the city of Izhevsk according to the old standards looks like this:
0.19 Gcal per person per month (this norm has already been canceled, but there is no other one, for example it will do) / 3.6 cubic meters per person per month (norm of hot water consumption) \u003d 0.05278 Gcal per 1 cubic meter. (so much heat is needed to heat 1 cubic meter of cold water to the standard temperature of hot water, which, recall, is 60 degrees C).
For a more accurate calculation of the amount of heat energy for heating water by the direct method based on physical quantities (and not in the opposite way based on the approved tariffs for hot water supply) - I recommend using template for calculating the tariff for hot water (REC UR)... In the calculation formula, among other things, the temperature of cold water in the summer and winter (heating) periods, the duration of these periods are used.
Tags: giga calorie, hot water
- We pay for hot water supply, the temperature is much lower than the standard. What to do?
- The duration of the DHW shutdown period established by the Rules is not illegal - the decision of the Supreme Court of the Russian Federation (2017)
- Initiative to establish fairer tariffs and metering methods for hot water consumption
- On the procedure for recalculating the amount of payment for heating and hot water supply in case of outages - clarification of Rospotrebnadzor for UR
- On metering the coolant in a closed heat supply system - letter of the Ministry of Construction of the Russian Federation dated 03.31.2015 No. 9116-OD / 04
- UR - On reducing payments for heating and hot water supply - letter from the Ministry of Energy UR dated 17.08.2015 No. 11-10 / 5661
- What is the standard period for calibrating a common building metering device for heating and hot water supply?
- Dirty hot water from the tap. Where to contact?
- Can the water meter in the apartment be turned up for the entire entrance? How to pay? Indications per month - 42 cubic meters
- The procedure for maintaining separate accounting of costs in the field of water supply and wastewater disposal - order of the Ministry of Construction of the Russian Federation dated January 25, 2014 No. 22 / pr
- payment for water and electricity in an apartment without accommodation
- heat calculation according to ODPU by 1/12
- Power supply
- Huge payments for a dorm room (17.3 sq.m.)
| Comments: (11) | |
| Hint: Share the link on social media if you want more responses / comments! | |
(or heat transfer).
Specific heat of a substance.
Heat capacity - This is the amount of heat absorbed by the body when heated by 1 degree.
The heat capacity of a body is indicated by a capital Latin letter FROM.
What determines the heat capacity of the body? First of all, from its mass. It is clear that heating, for example, 1 kilogram of water will require more heat than heating 200 grams.
And from the kind of substance? Let's make an experiment. Take two identical vessels and, pouring 400 g of water into one of them, and 400 g of vegetable oil into the other, we begin to heat them using identical burners. Observing the readings of the thermometers, we will see that the oil heats up quickly. To heat water and oil to the same temperature, the water must be heated longer. But the longer we heat the water, the more heat it receives from the burner.
Thus, to heat the same mass of different substances to the same temperature, different amounts of heat are required. The amount of heat required to heat a body and, therefore, its heat capacity depend on the kind of substance that makes up this body.
So, for example, to increase the temperature of water with a mass of 1 kg by 1 ° C, an amount of heat equal to 4200 J is required, and to heat the same mass of sunflower oil by 1 ° C, an amount of heat equal to 1700 J is required.
A physical quantity that shows how much heat is required to heat 1 kg of a substance by 1 ºС is called specific heat of this substance.
Each substance has its own specific heat, which is denoted by the Latin letter c and is measured in joules per kilogram-degree (J / (kg · ° C)).
The specific heat of the same substance in different states of aggregation (solid, liquid and gaseous) is different. For example, the specific heat capacity of water is 4200 J / (kg · ºС), and the specific heat capacity of ice is 2100 J / (kg · ° С); aluminum in the solid state has a specific heat equal to 920 J / (kg - ° C), and in the liquid state - 1080 J / (kg - ° C).
Note that water has a very high specific heat. Therefore, the water in the seas and oceans, when heated in summer, absorbs a large amount of heat from the air. Thanks to this, in those places that are located near large bodies of water, the summer is not as hot as in places far from water.
Calculation of the amount of heat required to heat a body or released by it during cooling.
From the above, it is clear that the amount of heat required to heat a body depends on the type of substance the body is made of (i.e., its specific heat capacity) and on the body's mass. It is also clear that the amount of heat depends on how many degrees we are going to increase the body temperature.
So, in order to determine the amount of heat required for heating a body or released by it during cooling, you need to multiply the specific heat of the body by its mass and by the difference between its final and initial temperatures:
Q = cm (t 2 - t 1 ) ,
where Q - quantity of heat, c - specific heat, m - body mass , t 1 - initial temperature, t 2 - final temperature.
When the body is heated t 2\u003e t 1 and therefore Q > 0 ... When cooling the body t 2 and< t 1 and therefore Q< 0 .
In case the heat capacity of the whole body is known FROM, Q determined by the formula:
Q \u003d C (t 2 - t 1 ) .