From this it was concluded that the red blood cell membrane consists of lipid molecules arranged in two layers. Model lipid membranes “Molecular organization of biological membranes”
11 State Budgetary Educational Institution of Higher Professional Education “Saratov State Medical University named after. IN AND. Razumovsky Ministry of Health of Russia"
1. Normal physiology: textbook / Ed. A.V. Zavyalova, V.M. Smirnova, 2011. – 368 p.
2. Normal physiology: textbook [N.A. Agadzhanyan, N.A. Barabash, A.F. Belov et al.] / Ed. prof. V.M. Smirnova. – 3rd ed. – M.: Publishing Center “Academy”, 2010. – 480 p.
3. Human physiology / V.F. Kirichuk, O.N. Antipova, N.E. Babichenko, V.M. Golovchenko, E.V. Ponukalina, I.V. Smyshleeva, L.K. Tokaev / Edited by V.F. Kirichuk. – 2nd ed. – Saratov: Publishing House of Saratov Medical University, 2009. – 343 p.
4. Physiology and pathophysiology of red blood: textbook. allowance / N.P. Chesnokova, V.V. Morrison, E.V. Ponukalina, T.A. Nevvazhay; under general ed. prof. N.P. Chesnokova. – Saratov: Publishing house Sarat. honey. University, 2013. – 80 p.
5. Hematological atlas / S. Lugovskaya, M.E. Postman. 3rd edition. – Moscow – Tver: Triada Publishing House LLC, 2011. – P. 3–23.
6. Cellular and molecular mechanisms of regulation of the hemostasis system in health and pathology: monograph / B.I. Kuznik. – Chita: Express Publishing House, 2010. – pp. 261–368.
7. Hematology / Edited by prof. O.A. Rukavitsina, A.D. Pavlova, E.F. Morshchakova and others - St. Petersburg: LLC "D.P.", 2007. - P. 29–34.
Features of the structural organization of the erythrocyte membrane
The red blood cell is surrounded by a plasma membrane, the structure of which is well studied and is identical to that of other cells. The cytoplasmic membrane of red blood cells includes a bilayer of phospholipids, while proteins either “float” on the surface of the membranes or penetrate the lipids, providing strength and viscosity to the membranes. The membrane area of one red blood cell is about 140 µm2.
Proteins account for approximately 49%, lipids - 44%, carbohydrates -7%. Carbohydrates are chemically bonded to either proteins or lipids and form glycoproteins and glycolipids, respectively.
The most important components of the erythrocyte membrane are lipids, including up to 48% cholesterol, 17-28% phosphotidylcholine, 13-25% sphingomyelin and a number of other phospholipids.
Phosphotidylcholine of the erythrocyte membrane carries a neutral charge and practically does not interact with positively charged Ca2+ channels, thereby ensuring the athrombogenicity of erythrocytes. Due to properties such as fluidity and plasticity, red blood cells are able to pass through capillaries with a diameter of ~ 3 μm.
Red blood cell membrane proteins are divided into peripheral and integral. Peripheral proteins include spectrin, ankyrin, protein 4.1, p55 protein, aducin, etc. The group of integral proteins includes fraction 3, as well as glycophorins A, B, C, O, E. Ankyrin forms a compound with p-spectrin. About 340 membrane and 250 soluble proteins were found in erythrocytes.
RBC plasticity is associated with phosphorylation of membrane proteins, especially band 4.1 proteins.
Protein fraction 4.2. - pallidin ensures the binding of the spectrin-actin-ankyrin complex to fraction 3, belongs to the group of transglutaminase proteins.
The contractile proteins of the erythrocyte membrane include p-actin, tropomodulin, stromatin and tropomyosin.
Glycophorins are integral proteins of the erythrocyte membrane that determine the negative charge that promotes the repulsion of erythrocytes from each other and from the vascular endothelium.
Protein 3 is the main actin protein that regulates the dephosphorylation of erythrocytes.
As mentioned above, the erythrocyte membrane is a complex complex, including lipids, proteins and carbohydrates organized in a certain way, which form the outer, middle and inner layers of the erythrocyte membrane.
Regarding the spatial arrangement of the various chemical components of the erythrocyte membrane, it should be noted that the outer layer is formed by glycoproteins with branched complexes of oligosaccharides, which are the terminal sections of group blood antigens. The lipid components of the outer layer are phosphatidylcholine, sphingomyelin and unesterified cholesterol. Lipids in the outer layer of the erythrocyte membrane play an important role in ensuring the constancy of the membrane structure and the selectivity of its permeability for various substrates and ions. Together with phospholipids, cholesterol regulates the activity of membrane-bound enzymes by changing membrane viscosity, and is also involved in modifying the secondary structure of enzymes. The cholesterol/phospholipid molar ratio in cell membranes in humans and many mammals is 0.9. An upward change in this ratio is observed in old age, as well as in some diseases associated with impaired cholesterol metabolism.
A decrease in the fluidity of the erythrocyte membrane and a change in its properties is also observed with an increase in the content of sphingomyelin,
The middle bilayer of the erythrocyte membrane is represented by hydrophobic “tails” of polar lipids. The lipid bilayer has pronounced fluidity, which is ensured by a certain ratio between saturated and unsaturated fatty acids of the hydrophobic part of the bilayer. Integral proteins, which include enzymes, receptors, and transport proteins, are active only if they are located in the hydrophobic part of the bilayer, where they acquire the spatial configuration necessary for activity. Therefore, any changes in the composition of the lipids of the erythrocyte membrane are accompanied by a change in its fluidity and disruption of the functioning of integral proteins.
The inner layer of the erythrocyte membrane, facing the cytoplasm, consists of the proteins spectrin and actin. Spectrin is a specific protein of erythrocytes; its flexible elongated molecules, binding to actin microfilaments and lipids of the inner surface of the membrane, form a kind of erythrocyte skeleton. A small percentage of lipids in the inner layer of the red blood cell membrane are phosphatidylethanolamine and phosphatidylserine. The mobility of proteins that hold the lipid bilayer depends on the presence of spectrin.
One of the important glycoproteins is glycophorin, which is contained on both the outer and inner surfaces of erythrocyte membranes. Glycophorin contains a large amount of sialic acid and has a significant negative charge. It is located unevenly in the membrane and forms areas protruding from the membrane, which are carriers of immunological determinants.
The structure and condition of the erythrocyte membrane, the low viscosity of normal hemoglobin provide significant plastic properties to erythrocytes, thanks to which the erythrocyte easily passes through capillaries, which have half the diameter of the cell itself, and can take on a wide variety of shapes. Another peripheral membrane protein of erythrocytes is ankyrin, which forms a compound with the P-spectrin molecule.
Functions of the erythrocyte membrane
The erythrocyte membrane provides regulation of the electrolyte balance of the cell due to active energy-dependent transport of electrolytes or passive diffusion of compounds along the osmotic gradient.
The erythrocyte membrane has ion-permeable channels for Na+, K+ cations, for O2, CO2, Cl- HCO3-.
The transport of electrolytes across the erythrocyte membrane and the maintenance of its membrane potential is ensured by energy-dependent Na+, K+, Ca2+ - ATPase systems.
The erythrocyte membrane is highly permeable to water with the participation of the so-called protein and lipid pathways, as well as anions, gaseous compounds, and poorly permeable to monovalent cations of potassium and sodium.
The protein pathway of transmembrane water transfer is ensured with the participation of the “band 3” protein that spans the erythrocyte membrane, as well as glycophorin.
The molecular nature of the lipid pathway for transporting water across the erythrocyte membrane is practically unknown. The passage of molecules of small hydrophilic nonelectrolytes through the erythrocyte membrane is carried out in the same way as water transfer, due to the protein and lipid pathways. The transfer of urea and glycerol across the erythrocyte membrane is ensured by enzymatic reactions.
A characteristic feature of the erythrocyte membrane is the presence of a powerful active transport system for monovalent anions (chlorine and fluorine), and divalent anions (SO42-, PO42-) due to carrier proteins.
The transport of organic anions across the erythrocyte membrane is ensured, like the transport of inorganic anions, with the participation of the “band 3” protein.
The erythrocyte membrane provides active transport of glucose, the kinetics of which is ensured by the Michaelis-Menten dependence. An important role in the transport of glucose across the erythrocyte membrane is assigned to the band 4.5 polypeptide (proteins with an MW of 55 kD are possible breakdown products of the band 3 polypeptide). It has been suggested that proteins that transport sugars in the erythrocyte membrane have a specific lipid environment.
The uneven distribution of monovalent cations in the erythrocyte-blood plasma system is maintained with the participation of an energy-dependent Na+ pump, which carries out the transmembrane exchange of erythrocyte Na+ ions for blood plasma K+ ions in a ratio of 3:2. In addition to the indicated transmembrane Na+/K+ exchange, the Na+ pump carries out at least four more transport processes: Na+ → Na+ exchange; K+→K+exchange; monovalent input of Na+ ions coupled with the output of K+.
The molecular basis of the Na+ pump is the enzyme Na+, K+ -ATPase - an integral protein firmly associated with membrane lipids, consisting of 2 polypeptide subunits with a MW of 80-100 kDa.
The transport system has 3 centers that bind Na+ ions, localized on the cytoplasmic side of the membrane. On the outside of the membrane on the transport system there are 2 binding centers for K+ ions. Membrane phospholipids play an important role in maintaining high enzyme activity.
The functioning of the Ca2+ pump is ensured by nucleotides, as well as high-energy compounds, mainly ATP, CTP, GTP, and to a lesser extent GTP and CTP.
As in the case of the Na+ pump, the functioning of the Ca2+ pump in erythrocytes is associated with manifestations of the activity of Ca2+, Mg2+ -ATPase. About 700 molecules of Ca2+, Mg2+ -ATPase are found in the membrane of one erythrocyte.
Along with barrier and transport functions, the erythrocyte membrane performs a receptor function.
The presence of receptors for insulin, endothelin, ceruloplasmin, α2-macroglobulin, α- and β-adrenergic receptors on the membrane of erythrocytes has been experimentally proven. On the surface of red blood cells there are receptors for fibrinogen, which have a fairly high specificity. Red blood cells also carry receptors for histamine, TxA2, and prostacyclin on their membrane.
Receptors for catecholamines are found in the erythrocyte membrane, which reduce the mobility of fatty acids in the lipids of erythrocyte membranes, as well as the osmotic stability of erythrocytes.
A restructuring of the erythrocyte membrane structure under the influence of low concentrations of insulin, human growth hormone, and prostaglandins E and E2 has been established.
In the membranes of erythrocytes, c-AMP activity is also high. With increasing concentrations of c-AMP in erythrocytes (up to 10-6 M), the processes of protein phosphorylation intensify, which in turn leads to a change in the degree of phosphorylation and permeability of erythrocyte membranes to Ca2+ ions.
The erythrocyte membrane contains isoantigens of various immunological reaction systems that determine the group affiliation of human blood according to these systems.
Antigenic structure of the erythrocyte membrane
The erythrocyte membrane contains various antigens of species, group and individual specificity. There are two types of erythrocyte isoantigens that determine the group specificity of human blood - A and B agglutinogens. Accordingly, two types of isoantibodies are found in plasma or serum - agglutinins α and β. Human blood does not contain the same agglutinogens and agglutinins. Their meeting and interaction can occur during transfusion of incompatible blood groups, leading to the development of agglutination and hemolysis of red blood cells.
As is known, blood group I (0) is characterized by the absence of agglutinogens A and B in erythrocytes, with the presence of agglutinins α and β in plasma or serum; it occurs in 40-50% of people in central European countries.
Blood group II (A) is characterized by the presence of agglutinogen A in the erythrocyte membrane, while the blood plasma contains β agglutinins. This blood type is common in 30-40% of people.
III (B) blood group is characterized by the presence of agglutinogen B in the membrane of erythrocytes, and in plasma or serum by the presence of type α agglutinins. This blood type occurs in approximately 10% of the population.
Blood group IV (AB) is characterized by the presence of fixed A and B agglutinogens in the membrane of red blood cells, while there are no natural agglutinins α and β in the blood plasma or serum. This blood type occurs in 6% of the population.
Genetic control of the antigenic system A, B, O of erythrocyte membranes is represented by genes O, H, A, B, localized in the long arm of the 9th pair of chromosomes.
Agglutinins α and β belong to the Ig M class, are natural antibodies, are formed in a child in the first year of life, reaching a maximum by 8 - 10 years.
The second place among the antigenic properties of erythrocyte membranes in clinical significance is occupied by the Rh - Hr system. The Rh factor was first discovered in 1940 by K. Landsteiner and A. Wiener; it is found in red blood cells in 85% of people of the white race. 15% of people lack these erythrocyte antigens. Currently, the lipoprotein nature of the antigens of this system has been established; there are about 20 of them; they form various combinations in the erythrocyte membrane. The most common rhesus antigens are 6 varieties: Rh0 (D), rh’ (C), rh’’ (E), Hr0 (d), hr’ (c), hr’’ (e). The most powerful antigen of this group is Rh0 (D).
Antibodies of the Rh and Hr system - anti-rhesusagglutinins are acquired, immune, are absent in the blood of Rh (-) people from the moment of birth, are synthesized during the first transfusion of Rh (+) blood to a Rh (-) recipient, as well as during the first pregnancy of a Rh (-) woman (+) fruit. During the first pregnancy, these antibodies are synthesized slowly over several months in a small titer, without causing serious complications in the mother and fetus. When a Rh-negative person comes into repeated contact with Rh-positive red blood cells, an Rh-conflict is possible. Antibodies of the Rh - Hr system belong to the Ig G class, so they easily penetrate the placental barrier, cause agglutination reactions and hemolysis of fetal red blood cells, which is accompanied by the development of hemolytic jaundice in newborns. In case of repeated transfusion of donor and recipient blood that is incompatible for Rh antigens, transfusion shock may occur.
Bibliographic link
Chesnokova N.P., Ponukalina E.V., Bizenkova M.N. LECTURE 2. FEATURES OF THE STRUCTURE AND FUNCTIONS OF THE ERYTHROCYTE MEMBRANE // Advances in modern natural science. – 2015. – No. 1-2. – P. 328-331;URL: http://natural-sciences.ru/ru/article/view?id=34842 (access date: 10/25/2019). We bring to your attention magazines published by the publishing house "Academy of Natural Sciences"
Liposomes, or phospholipid vesicles (bubbles), are usually obtained by swelling dry phospholipids in water or by injecting a solution of lipids into water. In this case, self-assembly of a bimolecular lipid membrane occurs. The minimum Gibbs energy corresponds to the closed spherical single-lamellar shape of the membrane. In this case, all non-polar hydrophobic tails are located inside the membrane and none of them comes into contact with polar water molecules (Fig. 1.11). However, non-spherical multilamellar liposomes consisting of several bimolecular layers are more often obtained - multilayer liposomes.
Rice. 1.11. Scheme of the structure of a single-layer liposome
The individual bimolecular layers of a multilayer liposome are separated by an aqueous medium. The thickness of the lipid layers is, depending on the nature of the lipids, 6.5 - 7.5 nm, and the distance between them is 1.5 - 2 nm. The diameter of multilayer liposomes ranges from 60 nm to 400 nm or more.
Single-layer liposomes can be obtained by various methods, for example, from a suspension of multilayer liposomes by treating them with ultrasound. The diameter of single-layer liposomes obtained by this method is 25-30 nm. Other methods for producing single-layer liposomes, including those with a diameter of up to 400 nm or more, have also been developed.
Liposomes are in some way a prototype of a cell. They serve as a model for studying various properties of cell membranes.
Liposomes have found direct application in medicine. For example, a drug can be enclosed inside liposomes and used as a phospholipid microcapsule to deliver the drug to specific organs and tissues. Liposomes are non-toxic (with the correct selection of lipids), are completely absorbed by the body, and are able to overcome some biological barriers. Thus, insulin enclosed in a liposome is protected from the action of digestive enzymes. Currently, the possibility of administering this drug in liposomes orally is being explored, which could save diabetic patients from the need for systematic injections. Work is underway to develop methods for liposomal therapy for tumors, enzyme deficiency, and atherosclerosis. The possibility of targeted delivery of a drug enclosed in liposomes to a diseased organ or even to a diseased area (in particular, to the affected area of the heart) is being studied.
To do this, a protein molecule - an antibody to the corresponding membrane antigen of the target organ - is attached to the liposome. Liposomes are carried through the bloodstream throughout the body and are retained once they are near the target organ.
Despite the attractive prospects of liposomal therapy, there are still many unresolved questions.
Rice. 1.12. Formation of a flat bilayer lipid membrane
Flat bilayer lipid membranes (BLMs) - another type of model membranes. Such membranes are produced on small holes with a diameter of about 1 mm in a plastic plate (for example, fluoroplastic) immersed in an aqueous environment. A drop of a lipid solution (in alcohol, chloroform, heptane or other solvents) is applied to the hole. The solvent diffuses from the solution into the water, leaving a film of lipid on the hole. This film thins spontaneously until a bimolecular layer about 6 nm thick is formed. Excess lipid collects in the form of a torus rim at the edges of the hole (Fig. 1.12).
Planar lipid membranes, along with liposomes, are widely used as models to study membrane electrical properties, membrane permeability, and other scientific studies. Using model membranes, a number of functions of biological membranes are studied, including barrier functions (for example, selectivity of permeability - good permeability for water and poor permeability for ions). Biological transport can be simulated by introducing carrier molecules into the model membrane.
test questions, tasks, assignments
1. The specific electrical capacitance of the axon membrane, measured by an intracellular microelectrode, turned out to be equal to 0.5 microfarads/cm 2. Using the formula of a flat capacitor, estimate the thickness of the hydrophobic layer of the membrane with a dielectric constant of 2.
2. How far does a phospholipid molecule travel on the surface of the erythrocyte membrane in 1 second as a result of lateral diffusion? The lateral diffusion coefficient is taken to be 10~ 12 m 2 /s. Compare with the circumference of a red blood cell with a diameter of 8 microns.
3. During the phase transition of membrane phospholipids from the liquid crystalline state to the gel, the thickness of the bilayer changes. How will the electrical capacitance of the membrane change? How will the electric field strength in the membrane change?
4. Using spin-labeled phospholipid molecules, a viscosity gradient across the membrane thickness was established. Describe the experiment. Where is the viscosity higher: at the surface of the membrane or in its center?
standard current control tests
1.1. Biological membrane thickness:
1. 10 A 3.0.1 µm
2. 10 nm 4. 10 µm
1.2. The fluid mosaic model of a biological membrane includes:
1. protein layer, polysaccharides and surface lipids!
2. lipid monolayer and cholesterol
3. lipid bilayer, proteins, microfilaments
4. lipid bilayer
1.3. The lipid part of the biological membrane is in the following physical state:
1. liquid amorphous
2. solid crystalline
3. solid amorphous
4. liquid crystal
1.4. Specific electrical capacitance of the axon membrane:
1. 0.5 10 -4 F/m 2 3. 0.5 10 -2 F/cm 2
2. 0.5 Yu -2 F/m 2 4. 0.5 10 -12 F/m 2
1.5. The characteristic time of transfer of a phospholipid molecule from one equilibrium position to another during their diffusion:
lateral flip-flop
1. 10 -7 – 10 -8 ~1 hour
2. 10 -10 – 10 -12 10 -7 – 10 -8 s
3. 1 – 2 hours 10 – 50 s
1.6. The phase transition of the lipid bilayer of membranes from the liquid crystalline state to the gel is accompanied by:
1. thinning of the membrane
2. membrane thickness does not change
3. thickening of the membrane
CHAPTER 2. TRANSPORT OF SUBSTANCES THROUGH BIOLOGICAL MEMBRANES
Living systems at all levels of organization are open systems. Therefore, the transport of substances through biological membranes is a necessary condition for life. Cell metabolic processes, bioenergetic processes, the formation of biopotentials, the generation of a nerve impulse, etc. are associated with the transfer of substances through membranes. Violation of the transport of substances through biomembranes leads to various pathologies. Treatment often involves the penetration of drugs through cell membranes. The effectiveness of a drug largely depends on the permeability of the membrane.
The concept of electrochemical potential is of great importance for describing the transport of substances.
The chemical potential of a given substance μ k is a value numerically equal to the Gibbs energy per one mole of this substance. Mathematically, the chemical potential is defined as the partial derivative of the Gibbs energy G with respect to the amount k-ro of the substance, at constant temperature T, pressure P and the amounts of all other substances m 1 (l≠k):
For a dilute solution of the concentration of substance C:
where μ Q is the standard chemical potential, numerically equal to the chemical potential of a given substance at its concentration of 1 mol/l in solution.
Electrochemical potential μ is a value numerically equal to the Gibbs energy G per one mole of a given substance placed in an electric field.
For dilute solutions
where F = 96500 C/mol is the Faraday number, Z is the charge of the electrolyte ion (in elementary charge units), φ is the electric field potential, T [K] is temperature.
Transport of substances across biological membranes can be divided into two main types: passive and active.
Active transport - transfer of molecules and ions, which occurs with the expenditure of chemical energy in the direction from smaller to larger values.
In this case, neutral molecules are transferred to an area of higher concentration, and ions are transferred against the forces acting on them from the electric field. Thus, active transport carries out the transfer of substances in the direction opposite to transport, which should occur under the influence of gradients (primarily concentration and electrical). Energy is obtained through the hydrolysis of molecules of a special chemical compound - adenosine triphosphoric acid (ATP). It has been experimentally established that the decay energy of one ATP molecule is sufficient to remove three sodium ions outside and introduce two potassium ions into the cell. The diagram of active transport is presented in Fig. 13.
Having captured a potassium ion from the external environment with one active center, and a sodium ion from the internal environment with the other, the system, consuming ATP, turns 180° inside the membrane. The sodium ion ends up outside the cell and is separated there, and the potassium ion gets inside and is also released, after which the protein molecule takes its original position, and everything starts all over again.
Due to active transport, the cell maintains a high concentration of potassium and a low concentration of sodium within itself. In this case, ions can move against their concentration gradient (analogy with a gas: pumping gas from a vessel with low pressure to a vessel with high pressure).
Fig. 13. Active transport scheme
Active transport of substances across biological membranes is of great importance. Due to active transport, concentration gradients, electrical potential gradients, pressure gradients, etc. are created in the body that support life processes, i.e., from the point of view of thermodynamics, active transport keeps the body in a non-equilibrium state and supports life.
The existence of active transport of substances through biological membranes was first proven in the experiments of Ussing (1949) using the example of the transfer of sodium ions through the skin of a frog (Fig. 14).
Rice. 14. Scheme of Ussing's experiment (A - ammeter, V - voltmeter, B - battery, P - potentiometer)
Ussing's experimental chamber, filled with normal Ringer's solution, was divided into two parts with freshly isolated frog skin. In Fig. 14, on the left is the outer mucosal surface of the skin, on the right is the inner serous. Flows of sodium ions through the skin of a frog were observed: from left to right from the outer to the inner surface and from right to left - from the inner to the outer surface.
A potential difference arose on the frog skin dividing Ringer's solution, with the inner side of the skin having a positive potential relative to the outer one. The installation had a voltage compensation unit, with the help of which the potential difference on the skin of the frog was set to zero, which was controlled by a voltmeter. In addition, the same ion concentration was maintained on the outside and inside. Under these conditions, if the transport of sodium ions through the skin of a frog was determined only by passive transport, then the flows of sodium ions should be equal to each other, and there would be no current in the circuit.
However, it was discovered that under experimental conditions (the absence of gradients of electrical potential and concentration) an electric current flows through the skin of the frog, therefore, a one-way transfer of charged particles occurs. It has been established that current flows through the skin from the external to the internal environment. Using the tagged atom method, it was shown that the inward flux of sodium is greater than the outward flux.
To do this, radioactive isotopes Na 22 were included in the left solution of the experimental chamber, and Na 24 in the right solution. The Na 22 isotope decays with the emission of hard γ quanta. The decay of Na 24 is accompanied by soft β-radiation. Registration of γ - and β - radiations showed that the Na 22 flux is greater than the Na 24 flux. These experimental data irrefutably indicated that the transport of sodium ions through the skin of a frog does not obey the passive transport equation. Therefore, active transfer takes place. Further experiments showed that depletion of ATP reserves in the frog's skin leads to a complete stop in the unidirectional flow of sodium ions.
3. The purpose of students’ activities in class:
The student must know:
1. The role of the membrane in the functioning of the cell.
2. Structure, structure and models of membranes.
3. Functions of the membrane.
4. Physical properties of membranes.
5. Fick's equation.
6. Nernst-Planck equation.
7. Types of passive transport of particles through the membrane.
8. Active transport of particles across the membrane.
The student must be able to:
1. Explain the structure of the membrane.
2. Explain artificial membrane models.
3. Explain the mechanism of passive transport across the membrane.
4. Explain the mechanism of active transport across the membrane.
5. Solve situational problems.
1. The structure of biological membranes.
2. Liquid mosaic model of the membrane.
3. Artificial membrane models.
4. Basic functions of the cell membrane.
5. Physical properties of membranes.
6. Transfer of molecules (atoms) across the membrane. Fick's equation.
7. Transfer of ions through membranes. Nernst-Planck equation.
8. Types of passive transport of molecules and ions through membranes.
9. Active transport. Ussing's experience.
10. Solving situational problems.
5.List of questions to check the initial level of knowledge:
1. What are biological membranes?
2. What is the basis of the membrane?
3. Why are physicochemical (artificial) membrane models used?
4. Describe the liquid mosaic model of the membrane.
5. What is lateral diffusion? flop-flop transition?
6. What are the main functions of the membrane and what are they?
7. Write down the Fick and Nernst-Planck equations. What processes do they describe?
8. What is called mobility?
9. What is passive transport? What types of passive transport are there?
10. What is active transport? How is it accomplished?
11. What is the importance of active transport of substances?
12. Explain the phenomena of matter and charge transfer through a membrane.
13. What happens if the cell is placed in clean water?
6 . List of questions to check the final level of knowledge:
1. Describe model lipid membranes. Where are they used?
2. Describe the physical properties of membranes.
3. During the phase transition of membrane phospholipids from the liquid crystalline state to the gel, the thickness of the bilayer changes. How will the electrical capacitance of the membrane change? How will the electric field strength in the membrane change?
4. Apply Fick's equation to a biological membrane.
5. Write and explain the Nernst-Planck equation.
6. Show that the Nernst-Planck equation reduces to the Fick equation for the diffusion of uncharged particles.
7. Describe the types of passive transport.
8. The permeability of cell membranes for water molecules is approximately 10 times higher than for ions. What happens if the concentration of an osmotically active substance (for example, Na+ ions) is increased in an isotonic aqueous solution containing red blood cells?
9. Describe Ussing’s experience.
7. Solve problems:
1. What distance does a phospholipid molecule travel on the surface of the erythrocyte membrane in 1 second as a result of lateral diffusion? The lateral diffusion coefficient is taken equal to 10 -12 m 2 /s. Compare with the circumference of a red blood cell with a diameter of 8 microns.
2. The specific electrical capacitance of the axon membrane, measured by an intracellular microelectrode, turned out to be equal to 0.5 μF/cm 2. Using the formula for a flat capacitor, estimate the thickness of the hydrophobic layer of a membrane with a dielectric constant of 2.
3. The thickness of the double layer at the membrane-electrolyte interface is characterized by the Debye radius δ . Define δ for the case when the electrolyte solution surrounding the membrane contains only potassium ions with a concentration of: 1) 10 -5 mol/l; 2) 10 -2 mol/l.
4. Find the Debye radius of screening created by calcium ions present in the solution with a concentration of 10 -5 mol/l and sodium ions with a concentration of 10 -4 mol/l. How will it change δ, if the solution contains only calcium ions at a concentration of 10 -4 mol/l?
5. The critical radius of a lipid pore in a membrane depends on the edge tension of the pore, the surface tension of the membrane and the membrane potential. Derive a formula for the critical pore radius. Calculate the critical pore radius in the absence of membrane potential. Assume the edge tension of the pore is 10 -11 N, the surface tension of the lipid bilayer is 0.3 mN/m.
6. Molar concentration of oxygen in the atmosphere with a= 9 mol/m. Oxygen diffuses from the surface of the insects' body inward through tubes called tracheae. The length of the average trachea is approximately h= 2 mm, and its cross-sectional area S= 2∙10 -9 m2. Assuming that the oxygen concentration inside the insect ( With) is half the oxygen concentration in the atmosphere, calculate the diffusion flux through the trachea. Oxygen diffusion coefficient D= 10 -5 m 2 /s.
7. The phospholipid bilayer likens a biological membrane to a capacitor. The membrane substance is a dielectric with a dielectric constant ε = 4. Potential difference between membrane surfaces U= 0.2 V at thickness d= 10 nm. Calculate the electrical capacitance of a 1 mm 2 membrane and the electric field strength in it.
8. The surface area of a cell is approximately equal to S=5∙10 -10 m 2. The specific electrical capacity of the membrane (capacity per unit surface) is Court= 10 -2 F/m2. In this case, the intercellular potential is equal to U= 70 mV. Determine: a) the amount of charge on the surface of the membrane; b) the number of monovalent ions forming this charge.
9. The enzyme Na + - K + - ATPase in the plasma membrane of the erythrocyte completed six cycles. How much sodium and potassium ions were actively transported? How much energy was consumed in this case if the hydrolysis of one mole of ATP is accompanied by the release of 33.6 kJ? The efficiency of the energy coupling process is considered 100%.
8. Independent work of students:
Using the textbook by Antonov V.F. et al. (§ 15.4.), familiarize yourself with the physical methods for determining the thickness of the membrane.
9. Chronograph of the training session:
1. Organizational moment – 5 min.
2. Analysis of the topic – 50 min.
3. Solving situational problems – 40 min.
4. Current knowledge control – 30 min.
5. Summing up the lesson – 10 min.
10. List of educational literature for the lesson:
1. Remizov A.N., Maksina A.G., Potapenko A.Ya. Medical and biological physics, M., Bustard, 2008, §§ 11.1, 11.2, 11.5, 11.6.
Blood and red blood cells. We continue to publish materials about blood.
What does a red blood cell look like? Under normal physiological conditions in the bloodstream, red blood cells have a biconcave shape with uniform thickenings along the edges and a central lighter part - pallor.
In a light optical examination, a normal erythrocyte routinely stained with acidic dyes has the shape of a disk with a diameter of 6.9-7.7 and up to 9.0 microns. Depending on their size, red blood cells are divided into micro- and macrocytes, but the bulk of them are represented by normocytes/discocytes.
Morphofunctional properties of erythrocytes
An erythrocyte is an anucleate biconcave cell with an average volume of 90.0 µm 3 and an area of 142 µm 2. Its maximum thickness is 2.4 microns, the minimum is 1 microns.
In the dried preparation, the average size of a red blood cell is 7.55 microns; 95% of its dry matter comes from the iron-containing protein hemoglobin and only 5% from other substances (other proteins and lipids). Such cells represent the absolute majority - over 85% - of the red blood cells of a healthy person.
The nuclear forms of the erythrocyte lineage are easily distinguished from most cells of the leukocyte lineage by the absence of granules in their cytoplasm (errors are possible only when identifying blast cells). Erythroblasts have more granular and dense nuclear chromatin.
The central cavity (pallor) of the erythrocyte disk accounts for from 35 to 55% of its surface, and in cross section the erythrocyte has the shape of a donut, which, on the one hand, ensures the preservation of hemoglobin and, on the other, allows the erythrocyte to pass even through the thinnest capillaries. The currently available models of the structure of an erythrocyte correspond to the idea of the specific properties of this cell, especially its shell, which, despite its sensitivity to deforming pressure, provides resistance to bending and an increase in the total surface.
Literature data indicate that the size and deformability of the erythrocyte membrane are their most important characteristics, which are associated with the normal functioning of these cells, including a high migration ability, participation in metabolic processes (primarily in the exchange of oxygen).
Changes in the microelastometric properties of erythrocytes and the “transformation” of discocytes into other morphological forms can be caused by various agents. Thus, the appearance of surface outgrowths leads to a decrease in the elasticity of the membrane, which may be due to opposing forces arising in the very process of deformation of the erythrocyte; deformation increases with decreasing ATP concentration in cells.
If the integrity of the cell membrane is violated, the erythrocyte loses its characteristic shape and turns into a spheroplast, which, in turn, is hemolyzed. The structure of the erythrocyte (discocyte) membrane is the same throughout; and despite the fact that depressions and bulges can appear in its various parts, changes in intra- or extracellular pressure with a spread of ±15% do not cause shrinkage of the entire cell, because it has a significant reserve of “anti-deformability”. The erythrocyte membrane has sufficient elasticity to withstand the effects of various factors that arise during the circulation of the erythrocyte through the bloodstream.
The composition of the erythrocyte membrane includes: phospholipids (36.3%), sphingomyelins (29.6%), cholesterol (22.2%) and glycolipids (11.9%). The first two elements are amphiphilic molecules in an aqueous environment, forming a characteristic lipid bilayer, which is also penetrated by integral protein molecules associated within the erythrocyte with its cytoskeleton.
Membrane lipids are in a liquid state and have low viscosity (only 10-100 times the viscosity of water). On the outer surface of the membrane there are lipids, sialic acid, antigenic oligosaccharides, and adsorbed proteins; the inner surface of the membrane is represented by glycolytic enzymes, sodium and calcium, ATPase, glycoproteins and hemoglobin.
The lipid bilayer of the membrane performs three functions: a barrier function for ions and molecules, a structural basis for the functioning of receptors and enzymes (proteins, glycoproteins, glycolipids) and a mechanical one. In the implementation of a specialized respiratory function - the transport of oxygen or carbon dioxide - the main role is played by membrane proteins, “built-in” into the lipid bilayer. Mature red blood cells are not capable of synthesizing nucleic acids and hemoglobin; They are characterized by a low level of metabolism, which ensures a fairly long life span of these cells (120 days).
As a red blood cell ages, its surface area decreases, while the hemoglobin content remains unchanged. It has been established that in “mature” age, red blood cells maintain a constant chemical composition for a long time, but as the cells age, the content of chemical substances in them gradually decreases. The erythrocyte cytoskeleton is formed and controlled by multigene and membrane-associated protein "families" that organize specialized membrane domains that support the function and form of this highly specialized cell.
Electric potential of a red blood cell
The erythrocyte membrane contains 50% protein, up to 45% lipids and up to 10% carbohydrates. On the surface of intact cells, the “network” distribution of charges is determined by a glycoprotein containing sialic (neutramic) acid, which determines up to 62% of the surface negative charge of the cell.
It is believed that each electric charge corresponds to 1 molecule of this acid. The loss of sialic acid from the surface of the erythrocyte leads to a decrease in its electrophoretic mobility (EPM) and suppression of cation transport. Consequently, on the surface of cells there is a “mosaic” of charges determined by cationic and anionic groups, the ratio of which determines the overall electrical charge of erythrocytes.
To maintain an optimal state of homeostasis, blood cells must have a stable charge. The high stability of EPP is ensured by a subtle mechanism of its regulation - the balance of lipid peroxidation (LPO) processes in erythrocyte membranes and the protective effect of the antioxidant system.
It has been empirically established that receptors for antibodies are located on the membrane of erythrocytes, and the presence of even a small amount of them on the surface can disrupt normal physiological functions in the body and change the EFP of erythrocytes. This may affect the level of hemoglobin in the latter, since the content of hemoglobin and EPP are strictly coordinated.
It is also necessary to take into account that under extreme influences on the body of negative factors, the products of lipid peroxidation affect the electrokinetic properties of erythrocytes. In turn, this is reflected in the rate of peroxide processes in their membranes.
Thanks to the electrostatic repulsion (“thrust” according to Chizhevsky) of similarly charged red blood cells, the latter move freely through the blood vessels, performing their oxygen transport function. Therefore, a violation of charge stability can be considered an integral indicator of pathological changes in the body.
1. From this it was concluded that the red blood cell membrane consists of lipid molecules arranged in two layers.
Apparently, this conclusion of Gorter and Grendel turned out to be correct only due to mutual compensation of errors, but in historical terms this work was of great importance, since since then the concept of a lipid bilayer as the structural basis of biological membranes has become dominant and, in fact, turned out to be correct.
The concept of a bimolecular lipid membrane was further developed in 1935 by the Davson-Danielli, or "sandwich" model, which proposed that proteins cover the surface of a lipid bilayer. It was an extraordinarily successful model, and over the next 30 years, numerous experimental data, especially those obtained using X-ray diffraction and electron microscopy, fully confirmed its adequacy. However, it was then discovered that membranes perform a huge variety of functions, and to explain this phenomenon, the original Davson-Danielli model was repeatedly modified.
Rapid progress in membranology, which resulted in the formation of modern concepts, was achieved largely due to advances in the study of the properties of membrane proteins. Electron microscopic studies using the freeze-cleavage method showed that globular particles were embedded in the membranes. Meanwhile, biochemists, using detergents, managed to dissociate the membranes into the state of functionally active “particles.” Data from spectral studies indicated that membrane proteins were characterized by a high content of α-helices and that they were likely to form globules rather than distributed as a monolayer on the surface of the lipid bilayer. The nonpolar properties of membrane proteins suggested the presence of hydrophobic contacts between the proteins and the internal nonpolar region of the lipid bilayer. At the same time, methods were developed that made it possible to reveal the fluidity of the lipid bilayer. Singer and Nicholson brought all these ideas together to create a fluid mosaic model. Within this model, the membrane is represented as a fluid phospholipid bilayer in which freely diffusing proteins are immersed. The previous Davson-Danielli model was static and successfully explained the structural data available at that time, obtained at a rather low resolution. At the same time, since 1970, much attention has been paid to the study of dynamic properties and their relationship with membrane functions. In recent years, the fluid mosaic model has also undergone modification, and this process will continue. In particular, it is now clear that not all membrane proteins diffuse freely in a liquid lipid bilayer. There is evidence of the existence of lateral domains in the membrane itself. The role of the cytoskeleton is also being carefully studied. It is becoming increasingly clear that some membrane regions appear to differ in structure from the classical lipid bilayer. Nevertheless, for the foreseeable future, the fluid mosaic model in its various modifications will serve as the conceptual basis for many membrane studies.
3. Membrane morphology
Two methods played an important role in elucidating the morphology of membranes: X-ray diffraction and electron microscopy. It was with their help that the correctness of the bilayer model was confirmed. However, it should be kept in mind that both of these methods face a number of limitations when elucidating a detailed picture of the molecular organization of membranes.
3.1 X-ray diffraction
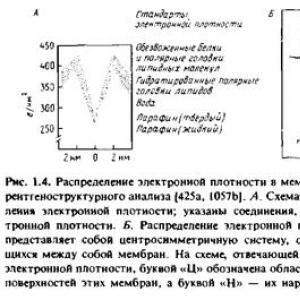
When studying highly ordered crystalline samples using X-ray diffraction, it is possible to obtain high-resolution structural information. In the case of poorly ordered drugs, the capabilities of this method are limited. Some specialized membrane systems already have a regular structure, and therefore they can be studied by X-ray diffraction methods. An example of this kind is the myelin sheath of peripheral nerve fibers; it is a membrane that, repeatedly wrapping around the axon, forms a regular system of concentric membrane structures. X-ray diffraction studies on myelin, carried out back in the 30s, confirm the adequacy of the bilayer membrane model. The same conclusion is led by the study of the outer segment of the retinal rods of vertebrates, which are natural ordered membrane systems, as well as artificially ordered systems that are formed during the collapse of membrane vesicles obtained from mitochondria and erythrocytes under centrifugation conditions. In all these cases, a similar distribution of electron density in the membrane was observed, shown in Fig. 1.4
To interpret X-ray diffraction data, it is necessary to determine not only the intensity of the reflections, but also their phases. In the case of regularly packed membrane systems, the task is greatly simplified, since these systems consist of repeating elements with central symmetry.

The data obtained show that the structure of all membranes is similar: they have a hydrophobic internal region with low electron density and two layers of polar groups with high electron density. X-ray diffraction data obtained for different membranes differ only slightly, despite large differences in their protein content. Although X-ray diffraction data provide some information about how the bulk of membrane proteins are located in the membrane, in general the X-ray diffraction method does not provide a detailed molecular picture.
Wilkins et al. noted in 1971 that X-ray diffraction could also be used to study aqueous dispersions of membranes and phospholipids. In this case, the reflections generated by the polar regions on both sides of the bilayer make it possible to find its thickness equal to the distance between the polar heads, and from the reflections generated by ordered hydrocarbon chains, the distance between these chains can be determined. And in this case, membrane preparations obtained from different sources gave a similar diffraction pattern, which confirms the universality of the bilayer model.
The inability to obtain a detailed molecular picture using the diffraction method limits the use of this method for studying biological membranes. However, it can be very useful in the study of ordered lipid-water systems.
3.2 Electron microscopy
Transmission electron microscopy of thin sections of myelin, and indeed all other membranes, reveals a characteristic three-layer structure consisting of two electron-dense bands separated by a gap of about 80 A. This picture is obtained largely as a result of treatment of the preparations with osmium tetroxide, usually used in this method. Robertson called the observed structure "unitary" to emphasize its versatility, and although the molecular mechanisms of osmium staining of membranes are unknown, this structure was seen as support for the bilayer membrane model. It is clear, however, that when preparing specimens for transmission electron microscopy, membranes may be subject to adverse effects. In particular, it is known that treatment with osmium tetroxide leads to significant loss of protein from the erythrocyte membrane. And although the three-layer structure observed in this case to some extent reflects the organization of bilayer membranes, more detailed information regarding the localization of proteins cannot be obtained by this method.
Some information about the location of membrane proteins was provided by new methods, which have now become “classical” - the methods of freezing-cleavage and freezing-etching. In these cases, the preparations are quickly frozen without exposing them to any damaging influences, as when obtaining thin sections. The drug preparation process includes the following operations.
After freezing, the sample, which is a suspension of cells or membranes, is cleaved using a knife at low temperature in a high vacuum. The forces generated during shearing lead to the formation of a shear passing through the sample. It turned out that when the cut plane passes through the membrane, the latter splits predominantly along its middle region and splits into two halves. As a result, the internal region of the membrane is exposed on the resulting cleavage planes.
If necessary, the sample is etched - the usual sublimation of ice in a vacuum is carried out. This allows for better visualization of the surface structures of cell membranes.
After this, a so-called replica is obtained from the exposed surface. It is this replica that is studied under an electron microscope. To obtain a replica, platinum is first sprayed onto the sample at an angle of about 45° to reveal the topological characteristics of the drug. The platinum replica is then given mechanical strength by coating it with a layer of carbon. After this, the drug is thawed, the replica floats up, and is caught using a special net.

The most characteristic structures observed when studying membranes by the freeze-cleavage method are numerous intramembrane particles with a diameter of 80 to 100 A, lying in the plane of membrane cleavages. Usually they are located chaotically, but sometimes they form groups. Numerous studies have shown that these particles may be membrane proteins. It is curious that electron microscopy of thin sections does not reveal such structures. The replicas obtained from the two halves of the split membrane are not always topologically complementary. This means that some particles are associated with only one half of the membrane. The freeze-cleavage data were widely used by Singer and Nicholson to develop the fluid mosaic model of membranes because they convincingly showed that globular proteins were not only located on the surface of the membrane, but also within the bilayer.
Figure 1.6 shows an electron micrograph of a preparation of proteoliposomes reconstructed from egg phosphatidylcholine and an unfractionated preparation of band 3 protein from the membrane of human erythrocytes; The drug was obtained by freezing - chipping.
Band 3 protein is a major protein component of the red blood cell membrane and is known to transport anions. If the phospholipid vesicles do not contain this protein, then the resulting frozen chip preparations have a smooth surface.
When the band 3 protein is incorporated into phospholipid vesicles, intramembrane particles appear on the cleaved surfaces, practically indistinguishable from particles observed in erythrocyte membranes. Moreover, at pH 5.5, the particles observed in the erythrocyte membrane aggregate, and this aggregation occurs as a result of the interaction of the band 3 protein with two other proteins, spectrin and actin.
The latter are components of the cytoskeleton located on the inner surface of the erythrocyte membrane. The reconstructed system consisting of band 3 protein and phosphatidylcholine behaves similarly, with particle aggregation observed in the presence of spectrin and actin at pH 5.5, but not at pH 7.6.

These data further strengthened the idea of membrane proteins as globular particles freely moving in the plane of the membrane. Interestingly, static micrographs of preparations obtained by the freeze-cleavage method helped researchers in studying the dynamic properties of membranes. As we will see, there are many proteins in membranes that cannot float freely in the lipid sea.
4. Isolation of membranes
Over the past three decades, it has become increasingly clear that the vast majority of cellular functions are carried out directly by membranes.
Both plant and animal cells are divided into compartments, and many cytoplasmic organelles, as was shown in section 1.1, are of a membrane nature.
In addition to the organelles characteristic of most cells, there are also specialized membrane systems, such as the sarcoplasmic reticulum of muscle cells, the myelin sheath of peripheral nerve fibers, thylakoid membranes of chloroplasts and disc membranes in retinal rods. Prokaryotic organisms also have membranes, although not as developed as eukaryotic ones.
Gram-positive bacteria, such as Bacillus subtilis, have only a cytoplasmic membrane, while gram-negative bacteria, such as Escherichia coli, also have an outer membrane, located on top of a thin peptidoglycan cell wall.
Some specialized organelles are also found in prokaryotic cells. Some viruses that are pathogenic to animals, such as enveloped viruses, have actual membranes, and such membranes have proven extremely interesting to study.
The study of membranes, as a rule, involves their purification, and each type of membrane has its own conditions for preparative isolation.
So, if you want to examine the plasma membrane of any cells, then you first need to isolate these cells from the tissue. Then you need to find the optimal conditions for destroying the cells and separating the membranes of interest from other cellular components. The purity criteria of the isolated membranes deserve special attention.
4.1 Cell destruction
It is advisable to choose a technique that allows you to effectively destroy the cells themselves while maintaining the structure of the membranes to be isolated. For many animal cells, a relatively mild procedure such as homogenization in a glass-walled Dounce or Potter-Elweheim homogenizer with a Teflon pestle can be used. In this case, the cells are destroyed due to shear forces that arise when the suspension is forced through a narrow gap between the Teflon pestle and the glass wall of the homogenizer. With this treatment, the plasma membrane is “torn off” and the connections between various organelles are destroyed while maintaining the integrity of the organelles themselves. Using this procedure, it is also possible to separate specialized regions of the plasma membrane from each other, for example, the basolateral or apical regions of the membrane of epithelial cells. It is desirable to work under conditions where organelle integrity is maintained to minimize the possibility of release of hydrolytic enzymes and to facilitate subsequent membrane separation operations.
To destroy cells that have a wall, more stringent methods are required. Sometimes, before cells are destroyed, they are first treated with enzymes that break down components of the cell wall to facilitate its subsequent destruction. For example, treatment with Tris-EDTA buffer and lysozyme is used to destroy E. coli cells. More stringent techniques include grinding the cells, treating them with ultrasound, and extruding them. Rubbing is usually carried out in the presence of various abrasive materials - sand, aluminum oxide or glass beads. Small volumes of material can be ground in a mortar and pestle, but for larger volumes special mechanical devices should be used. Bacterial cells are often destroyed using ultrasound. It is believed that in this case the destruction occurs under the influence of shear forces resulting from cavitation. The same forces occur when pressing a cell suspension through a small hole, for example, when destroying cells using a French press. There are many varieties of the listed methods, and their choice depends on the characteristics of the membrane system that is being studied.
It should be noted that membrane fragments obtained during cell destruction usually spontaneously form vesicles. Examples include:
1) microsomes obtained from the plasma membrane, endoplasmic reticulum or specialized systems such as the sarcoplasmic membrane;
2) submittedmitochondrial particles from the inner mitochondrial membrane;
3) synaptosomes formed when nerve endings are torn off in the area of synaptic contacts;
4) bacterial membrane vesicles formed from the plasma membrane of E. coli. Vesicles are also formed from other membrane systems, for example from the membranes of the Golgi apparatus. Their size in most cases strongly depends on the method of cell destruction. This is especially important since the size of the vesicles largely determines the rate of their sedimentation during centrifugation and their behavior during the subsequent stages of membrane purification. Some membranes do not form vesicles, in particular the membranes of the lateral surfaces of animal cells in contact with each other. When such cells are destroyed, a pair of adjacent membrane fragments, held together by the contact area, separates. The presence of such contacts prevents the fragments from closing into vesicles, so the membranes are released in the form of plates or ribbon-like structures.
The correct choice of medium is also of great importance when destroying cells. For example, to maintain the closure of membrane organelles, one should use a medium that is isosmotic to their internal contents. Most often, a sucrose solution in a concentration of 0.25-0.30 M is used for this. In some cases, it is better to use sorbitol and mannitol. It should be noted that maintaining isotonicity also plays an important role at subsequent stages of preparative isolation of intact organelles.
4.2 Membrane separation
Currently, centrifugation is most often used to separate membranes. Membrane particles can be classified according to their sedimentation rate or buoyant density. The first method is called zonal centrifugation and separation occurs according to S values, and the second is isopycnic centrifugation and separation occurs under equilibrium density conditions. In practice, some hybrid of these two methods is usually used. Figure 1.7 shows the position of some subcellular units on the “S-g” coordinate plane.
The abscissa axis shows the sedimentation coefficients of particles, and the ordinate axis shows the density.

The principle of separation by sedimentation rate can be easily understood by comparing the S values for different fractions. For example, nuclei have relatively high S values, i.e. their sedimentation rate is significantly higher than that of most other subcellular organelles. Nuclei can be selectively pelleted by centrifugation of the cell homogenate, leaving all other organelles in the supernatant. At the same time, smooth and rough endoplasmic reticulum cannot be separated using zonal centrifugation.
Differences in their density are often used to isolate different membrane fractions from a cell homogenate. For this purpose, centrifugation is carried out in a density gradient. Sucrose is most often used to create a density gradient, but this method has serious drawbacks. To obtain the density required to separate the various membrane fractions, it is necessary to prepare solutions with high concentrations of sucrose, which have a high viscosity and are also hypertonic. The introduction of subcellular organelles into a hypertonic sucrose solution leads to their dehydration, and subsequent bringing of the solution to isotonic conditions is often accompanied by lysis and damage to the organelles. Another problem is that many membrane organelles are permeable to sucrose. This can also lead to osmotic destruction of organelles. The penetration of sucrose into separated membrane organelles can change their effective density.
Table 1.1. Physical time increasingly uses other media to create a density gradient. Some of these environments are listed in Table 1.1
To solve these problems, the latest properties of gradient media.
1. Ficoll. High molecular weight hydrophilic polymer of sucrose, which can be used to obtain solutions with a Density of up to 1.2 g/ml. Its main advantage is the low osmotic pressure of solutions compared to solutions with an equivalent concentration of sucrose. Thanks to this, it is possible to create solutions that are isotonic throughout range of concentrations due to the additional inclusion of sucrose or physiologically acceptable salts in the medium.The disadvantages are the high viscosity of the resulting solutions and the significantly nonlinear dependence of viscosity and osmolarity on concentration.
2. Metrizamide. Triiodo-substituted glucose benzamide Metrizamide solutions have a higher density than Ficoll solutions at the same concentrations. The main advantage of metrizamide solutions is their very low viscosity, which allows for faster separation. A 35% metrizamide solution has an almost physiological osmolarity, so that most of the membrane separation operations can be carried out without exposing them to hypertonic solutions. Sodium metrizoate is a compound related to metrizamide with similar properties, with the only difference being that its solution is isotonic at a concentration of about 20%. Sodium metrizoate is used primarily for isolating intact cells. Nicodenz is also a derivative of triiodobenzoic acid, but has three hydrophilic side chains. When centrifuged, it quickly forms its own density gradient; used to isolate subcellular organelles.
Percoll. A colloidal suspension of silica gel, the particles of which are coated with polyvinylpyrrolidone. This coating reduces the toxic effects of silica gel. The main advantage of Percoll is that it does not penetrate biological membranes, and its solutions have low viscosity and low osmolarity. Due to the large particle size, centrifugation of the Percoll solution at moderate speeds results in the formation of a density gradient. Therefore, separation usually occurs very quickly. The medium used for centrifugation can be isotonic throughout its volume due to the inclusion of salts or sucrose. It is not difficult to create a gentle gradient, which allows for a very effective separation of membrane fractions based on their buoyant density.
Sorbitol and mannitol. These substances are sometimes used instead of sucrose because, according to published data, they penetrate some biological membranes less well than sucrose.
Note that glycerol is not used to create a density gradient, since it cannot achieve high enough density values. Alkali metal salts, such as CsCl, are used only when high-density solutions are required. But it should be borne in mind that in the concentrations required to create equilibrium density, these salts often have a damaging effect on membrane organelles.
Other methods are also used to isolate membranes from cell homogenates, although not as frequently as centrifugation.
1. Phase distribution. In this case, the separation of membrane particles occurs in accordance with their surface properties. For this purpose, two immiscible layers of aqueous solutions of various water-soluble polymers are formed. An example is a mixture of polyethylene glycol dextran and dextran ficoll. Membrane particles are separated according to their affinity for these phases. The latter can be selected so as to separate membranes according to their surface charge or hydrophobicity.
Continuous free flow electrophoresis. In this case, the separation of particles occurs in accordance with their electrical charge. The drug to be separated is continuously introduced into a thin layer of buffer flowing down a vertical wall. In this case, an electric field is applied perpendicular to the direction of flow. Thus, the electrophoretic separation of particles occurs across the flowing buffer, which collects at the bottom of the chamber in the form of separate fractions.
Affinity adsorption. The separation is based on a biospecific interaction between the membrane components and the solid phase. With the discovery of monoclonal antibodies, it became possible to create preparative techniques based on the use of specific antigenic components for isolating membranes. The resulting antibodies can be covalently attached to a solid support and with their help carry out specific binding of the corresponding membranes. This method is most often used to isolate membrane proteins. One of the problems that arises here is related to the selection of membrane elution conditions that would not cause protein denaturation.
Method based on the use of silica gel microgranules. Typically, plasma membranes account for no more than 1°7o of the total mass of all membranes of eukaryotic cells. Therefore, the isolation of absolutely pure plasma membranes is fraught with great difficulties. One approach that has been developed specifically for isolating plasma membranes is based on the use of cationized silica gel microbeads. These granules are strongly adsorbed to the outer surface of the plasma membrane of intact cells, and the fraction of plasma membranes associated with granules is easily separated along the sucrose density gradient from other membranes due to the higher density of granules. The peculiarity of this method is that in the resulting preparation the plasma membrane with its inner surface is turned into solution.
4.3 Purity criteria for membrane fractions
Perhaps the most objective criterion for the purity of the isolated membrane fraction is the presence in it of any unique component that is contained only in this membrane or is predominant in it. Typically, such components are enzymes, which in this case are called markers. A list of marker enzymes that are used to control the purity of membrane fractions is given in Table 1.2 When determining the activity of the enzyme, it should be taken into account that it may be in a latent form, for example, due to the fact that it is localized on the inner surface of secreted membrane vesicles. Other problems associated with assessing the purity of isolated membranes are discussed in the review. It should be noted that the recommended methods in most cases are quite well established and standardized.
In some cases, more convenient membrane markers are not enzymes, but specific receptors for lectins, hormones, toxins or antibodies. If the systems under study are well characterized, then the purity of the membrane fraction can be judged by its protein composition, determined using polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. For example, the outer membrane of gram-negative bacteria has a characteristic set of polypeptides that are not found in the cytoplasmic membrane.
Table 1.2 Markers used to control the purity of membrane fractions isolated from mammalian cells "
| Membrane fraction | Marker enzyme |
| Plasma membranes | 5 "-Nucleotidase |
| Alkaline phosphodiesterase | |
| Na */K + -ATPase (basolateral- |
|
| epithelial membrane | |
| cells) | |
| Adenylate cyclase (basal | |
| hepatocyte membrane) | |
| Aminopeptidase (membrane | |
| brush border epithelium) | |
| Mitochondria (internal | Cytochrome c oxidase |
| membrane) | Succinate-cytochrome c-oxido- |
| reductase | |
| Mitochondria (outer | Monoamine oxidase |
| membrane) | |
| Lysosomes | Acid phosphatase |
| 0-Galactose | |
| Peroxisomes | Catalase |
| Urate oxidase | |
| D-amino acid oxidase | |
| Device membranes | Galactosyltransferase |
| Golgi | |
| Endoplasmic | Glucose-6-phosphatase |
| reticulum | Choline phosphotransferase |
| NADPH-cytochrome c-oxido- | |
| reductase | |
| Cytosol | Lactate dehydrogenase |
Other criteria by which the purity of membranes can be judged include their morphology, revealed by electron microscopy, and features of the chemical composition. For example, fractions representing the plasma membrane, Golgi apparatus, or mitochondria can be identified by their morphology. In some cases, the drug is characterized by its cholesterol content. For example, mitochondrial membranes contain much less cholesterol than the membranes of the Golgi apparatus and plasma membranes.
There are detergent molecules per micelle. A fairly limited range of detergents is used in membrane studies. In table 1 presents those that are most often used for solubilization and reconstruction of membranes. These detergents are characterized by fairly high CMC values (10-4-10-2 M) and the fact that they belong to the category of so-called soft detergents, that is, such...

The formation of a bilayer is a special property of lipid molecules and occurs even outside the cell. The most important properties of a bilayer: - ability to self-assemble - fluidity - asymmetry. 1.2. Although the basic properties of biological membranes are determined by the properties of the lipid bilayer, most specific functions are provided by membrane proteins. Most of them penetrate the bilayer in the form of a single...