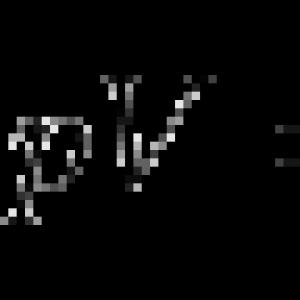Basics of molecular kinetic theory of MTT. Molecular kinetic theory
Molecular kinetic theory (Abbreviated ICT) - the theory, which arose in the XIX century and considering the structure of the substance, mainly gases, from the point of view of three major approximately correct positions:
all bodies consist of particles: atoms, molecules and ions;
particles are in continuous chaotic movement (thermal);
particles interact with each other by absolutely elastic collisions.
The ICT has become one of the most successful physical theories and was confirmed by a number of experienced facts. The main evidence of the provisions of the ICT steel:
Diffusion
Brownian motion
The change aggregate states Substances
On the basis of the ICT, a number of sections of modern physics are developed, in particular, physical kinetics and statistical mechanics. In these sections of physics are studied not only molecular (atomic or ionic) systems that are not only in the "thermal" movement, and interacting not only through absolutely elastic collisions. The term of the same molecular kinetic theory in modern theoretical physics is already practically not used, although it is found in textbooks at the rate of general physics.
Perfect Gas. - mathematical model gasin which it is assumed that: 1) potential energy interaction molecules can be neglected compared to their kinetic energy; 2) The total volume of gas molecules is negligible. Between molecules do not apply the forces of attraction or repulsion, the collision of particles between themselves and with the walls of the vessel absolutely elasticAnd the interaction time between molecules is negligible compared to the average time between collisions. In the extended model of the perfect gas particle, from which it consists, also have a form in the form of elastic spheres or ellipsoid, which makes it possible to take into account the energy of not only progressive, but also of the rotational and oscillatory movement, as well as not only central, but also non-central collisions of particles, etc.
Distinguish the classic ideal gas (its properties are derived from the laws of classical mechanics and are described boltzmann statistics) and quantum ideal gas (properties are determined by the laws of quantum mechanics, are described by statistics Fermi - Diraka or Bose - Einstein)
Classic perfect gas
The volume of the ideal gas linearly depends on the temperature at constant pressure
The properties of the ideal gas based on molecular-kinetic representations are determined on the basis of the physical model of the ideal gas, which adopted the following assumptions:
In this case, the gas particles move independently of each other, the gas pressure on the wall is equal to the full pulse transmitted when the particles collide with the wall per unit of time, internal energy - The sum of the energy of particles of gas.
According to the equivalent wording, the perfect gas is such a gas that simultaneously obeys boyle's law - Mariotta and Gay Loussaka , i.e:
where - the pressure is the absolute temperature. The properties of the perfect gas are described mendeleev - Klapairone Equation
![]() ,
,
where - , - weight, - molar mass.
where - concentration of particles, - permanent Boltzmanna.
For any ideal gas fairly majer's ratio:
![]()
where - universal gas constant- molar heat capacity At constant pressure, - molar heat capacity at a constant volume.
Statistical calculation of the distribution of speeds of molecules was performed by Maxwell.
Consider the result obtained by Maxwell in the form of a graph.
Gas molecules at their movement are constantly faced. The speed of each molecule during collision changes. It may increase and decrease. However, the riconductic speed remains unchanged. This is due to the fact that in a gas, which is at a certain temperature, a certain stationary, non-changing distribution of molecules in terms of speeds, which is subordinate to a certain statistical law is established. The speed of a separate molecule over time may vary, however, the proportion of molecules with speeds in some speeds remains unchanged.
It is impossible to raise the question: how many molecules have a certain speed. The fact is that, at least the number of molecules is very large in any even small volume, but the number of speed values \u200b\u200bis arbitrarily large (as numbers in a sequential row), and it may happen that no molecule has a given speed.
|
|
Based on the experience of the Stern, it can be expected that the greatest number of molecules will have some average speed, and the share of fast and slow molecules is not very large. Required measurements showed that the proportion of molecules referred to the speed interval Δ v.. It has the appearance shown in Fig. 3.3. Maxwell In 1859, theoretically, on the basis of the theory of probability, determined this function. Since then, it is called the distribution function of molecules in speeds or Maxwell law.

We will withdraw the function of the distribution of molecules of perfect gas at speeds 
 - speed interval near speed
- speed interval near speed  .
.
 - the number of molecules whose velocities lie in the interval
- the number of molecules whose velocities lie in the interval  .
.
 - The number of molecules in the volume under consideration.
- The number of molecules in the volume under consideration.
 - The angle of molecules whose velocities belong to the interval
- The angle of molecules whose velocities belong to the interval  .
.
 - the proportion of molecules in the unit speed range near speed
- the proportion of molecules in the unit speed range near speed  .
.
 - Formula Maxwell.
- Formula Maxwell.
Using Maxwell's statistical methods, we obtain the following formula:
 .
.
 - Mass of one molecule,
- Mass of one molecule,  - Permanent Boltzmann.
- Permanent Boltzmann.
The most suitable speed is determined from the condition  .
.
Solving receiving  ;
;
 .
.
Denote by h / s  .
.
Then  .
.
Calculate the share of molecules at a predetermined speed range near the specified speed in the specified direction.
 .
.
 .
.
 - the proportion of molecules that have speeds in the interval
- the proportion of molecules that have speeds in the interval  ,
,
 ,
,
 .
.
Developing the ideas of Maxwell Bolzman calculated the distribution of molecules in speeds in the power field. In contrast to the distribution of Maxwell in the distribution of the Boltzmann instead of the kinetic energy of molecules, the amount of kinetic and potential energy appears.
In the distribution of Maxwell:  .
.
In the distribution of Boltzmann:  .
.
In gravitational field 
 .
.
For the concentration of ideal gas molecules, the formula is:


 and
and  respectively.
respectively.
 - The distribution of the Boltzmann.
- The distribution of the Boltzmann.
 - concentration of molecules at the surface of the Earth.
- concentration of molecules at the surface of the Earth.
 - concentration of molecules at height
- concentration of molecules at height  .
.
Heat capacity.
The heat capacity is called a physical value equal to the ratio
 ,
,
 .
.
The heat capacity of one pray - molar heat capacity
 .
.
Because  - Process function
- Process function  T.
T.  .
.
Considering 
 ;
;

 ;
;





 .
.
 - Formula Mayer.
- Formula Mayer.
So The task of calculating the heat capacity is reduced to finding  .
.
 .
.

For one praying: 
 From here
From here  .
.
Double gas (O 2, N 2, CL 2, CO, etc.).
(model of hard dumbbell).
Complete number of degrees of freedom:
 .
.
Then  T.
T. 
 ;
;
 .
.
This means that the heat capacity should be constant. At the same time, the experience says that heat capacity depends on temperature.
Upon decreasing temperature, the oscillatory degrees of freedom, and then the rotational degrees of freedom.
According to the laws of quantum mechanics, the energy of a harmonic oscillator with a classic frequency can only receive a discrete set of values
Multiatomic gases (H 2 O, CH 4, C 4 H 10 O, etc.).
 ;
;
 ;
;
 ;
;
 Compare theoretical data with experienced.
Compare theoretical data with experienced.
It's clear that  2 atomic gases equals
2 atomic gases equals  but varies at low temperatures against the theory of heat capacity.
but varies at low temperatures against the theory of heat capacity.
Such a course of curve  from
from  Specifies to "freezing" degrees of freedom. On the contrary, additional degrees of freedom are connected at high temperatures. These data are questioned by the theorem on the uniform distribution. Modern physics allows us to explain the addiction
Specifies to "freezing" degrees of freedom. On the contrary, additional degrees of freedom are connected at high temperatures. These data are questioned by the theorem on the uniform distribution. Modern physics allows us to explain the addiction  from
from  Using quantum representations.
Using quantum representations.
Quantum statistics eliminated the difficulties in explaining the dependence of the heat capacity of gases (in particular ductomic gases) on temperature. According to the provisions of quantum mechanics, the energy of the rotational motion of molecules and the energy of atomic oscillations can take only discrete values. If the heat of the heat movement is significantly less than the difference in the neighboring energy levels (), then when the molecules collide, the rotational and oscillatory degrees of freedom are practically not excited. Therefore, at low temperatures, the behavior of the dihythomic gas is similar to the behavior of the same one. Since the difference between adjacent rotational levels of energy is significantly less than between adjacent oscillatory levels ( ![]() ), with increasing temperatures, rotational degrees of freedom are initially excited. As a result, heat capacity increases. With a further increase in temperature, oscillatory degrees of freedom are excited, and a further increase in heat capacity occurs. A. Einstein, approximately believed that the oscillations of the atoms of the crystal lattice are independent. Using the crystal model as a set independently fluctuating with the same frequency of harmonic oscillators, it created a high-quality quantum theory of the heat capacity of the crystal lattice. This theory was subsequently developed by Debay, who took into account that the oscillations of atoms in the crystal lattice are not independent. Having considered the continuous spectrum of oscillators frequencies, Deba showed that the main contribution to the average energy of the quantum oscillator is made of oscillations at low frequencies corresponding to elastic waves. The thermal excitation of the solid can be described in the form of elastic waves propagating in the crystal. According to the corpuscular-wave dualism of the properties of the substance, the elastic waves in the crystal are compared with fontone quasiparticlesHolding energy. Fonon - the energy quantum of an elastic wave, which is an elementary excitation, leading itself like a microparticle. As quantization of electromagnetic radiation led to the representation of photons, so quantization of elastic waves (as the result of thermal oscillation of solids molecules) led to a representation of phonons. The energy of the crystal lattice consists of the energy of phonon gas. Quasiparticles (in particular phonons) are very different from conventional microparticles (electrons, protons, neutrons, etc.), as they are associated with the collective movement of many particles of the system.
), with increasing temperatures, rotational degrees of freedom are initially excited. As a result, heat capacity increases. With a further increase in temperature, oscillatory degrees of freedom are excited, and a further increase in heat capacity occurs. A. Einstein, approximately believed that the oscillations of the atoms of the crystal lattice are independent. Using the crystal model as a set independently fluctuating with the same frequency of harmonic oscillators, it created a high-quality quantum theory of the heat capacity of the crystal lattice. This theory was subsequently developed by Debay, who took into account that the oscillations of atoms in the crystal lattice are not independent. Having considered the continuous spectrum of oscillators frequencies, Deba showed that the main contribution to the average energy of the quantum oscillator is made of oscillations at low frequencies corresponding to elastic waves. The thermal excitation of the solid can be described in the form of elastic waves propagating in the crystal. According to the corpuscular-wave dualism of the properties of the substance, the elastic waves in the crystal are compared with fontone quasiparticlesHolding energy. Fonon - the energy quantum of an elastic wave, which is an elementary excitation, leading itself like a microparticle. As quantization of electromagnetic radiation led to the representation of photons, so quantization of elastic waves (as the result of thermal oscillation of solids molecules) led to a representation of phonons. The energy of the crystal lattice consists of the energy of phonon gas. Quasiparticles (in particular phonons) are very different from conventional microparticles (electrons, protons, neutrons, etc.), as they are associated with the collective movement of many particles of the system.
Phonons cannot occur in vacuo, they exist only in a crystal.
The phonon pulse has a kind of property: when the phonons collishes in the crystal, their impulse can be transmitted by discrete portions to the crystal lattice - the pulse is not saved. Therefore, in the case of phonons, they say quasi-pulse.
Phonons have a spin equal to zero, and are bosons, and therefore phonon gas is subordinate to the Bose Einstein statistics.
Phonons can be emitted and absorbed, but their number is not preserved constant.
The use of Bose Einstein statistics to phonon gas (gas from independent bose particles) led the debt to the next quantitative conclusion. At high temperatures, which are many more characteristic temperatures of the debt (classical region), the heat capacity of solids is described by the law of dullette and PH, according to which the molar heat capacity of the chemically simple bodies in the crystalline state is the same ![]() And does not depend on temperature. At low temperatures, when (quantum region), the heat capacity is proportional to the third degree of thermodynamic temperature: the characteristic temperature of the debt is:, where is the limit frequency of elastic oscillations of the crystal lattice.
And does not depend on temperature. At low temperatures, when (quantum region), the heat capacity is proportional to the third degree of thermodynamic temperature: the characteristic temperature of the debt is:, where is the limit frequency of elastic oscillations of the crystal lattice.
The central concept of this topic is the concept of a molecule; The complexity of his assimilation by schoolchildren is related to the fact that the molecule is an object directly unobservable. Therefore, the teacher must convince ten-graders in the reality of the microworld, in the possibility of his knowledge. In this regard, much attention is paid to the consideration of experiments proving the existence and movement of molecules and allowing them to calculate their main characteristics (classical experiences perrin, Rayleigh and Stern). In addition, it is advisable to acquaint students with the calculated methods for determining the characteristics of molecules. When considering proof of the existence and movement of molecules, students are told about the observations of the brown disorderly movement of small suspended particles, which did not stop during the entire observation time. At that time, it was not possible to properly explain the reasons for this movement, and only after almost 80 years old A. Einstein and M. Smillukhovsky built, and J. Perenn was experimentally confirmed by the theory of Brownian movement. From the consideration of Brown experiments, it is necessary to draw the following conclusions: a) the movement of Brownian particles is caused by blows of a substance molecules in which these particles weighed; b) Brownian movement continuously and randomly, it depends on the properties of a substance in which particles weighed; c) the movement of Brownian particles allows you to judge the movement of the molecules of the medium in which these particles are located; d) Brownian movement proves the existence of molecules, their movement and continuous and chaotic character of this movement. Confirmation of this nature of the movement of molecules was obtained in the experience of French physics of Duneye (1911), which showed that gas molecules move in various directions and in the absence of collisions their movement is straightforward. Currently, the fact of the existence of molecules has no doubt. The development of equipment made it possible to directly observe large molecules. The story about Brownian movement is advisable to accompany the demonstration of the model of the Brownian movement in a vertical projection using a projection lantern or a codecope, as well as a film filming of the Brownian Movement Film Film from the movie "Molecules and Molecular Movement". In addition, it is useful to monitor the Brownian movement in fluids using a microscope. The drug is made from a mixture of equal parts of two solutions: a 1% solution of sulfuric acid and a 2% aqueous hydraulic solution of hyposulfite. As a result of the reaction, sulfur particles are formed, which are in a suspended solution. Two drops of this mixture are placed on the slide and observe the behavior of sulfur particles. The drug can be made of a strongly diluted solution of milk in water or from watercolor paint solution in water. When discussing the issue of dimensions of molecules, the essence of the experience of R. Rayleigh, which is as follows: to the surface of the water, poured into a large vessel, placed a drop of olive oil. The drop spreads over the surface of the water and forms a round film. Ralea suggested that when the drop stops spread, its thickness becomes equal to the diameter of one molecule. Experiments show that the molecules of various substances have different dimensions, but to estimate the dimensions of molecules take a value equal to 10 -10 m. In the class, you can do a similar experience. To demonstrate the estimated method for determining the dimensions of molecules, an example of calculating the diameters of molecules of various substances by their densities and constant avogadro is given. To present the small dimensions of molecules to schoolchildren is difficult, this is useful to bring a number of comparative examples. For example, if you increase all sizes in as many times so that the molecule is visible (i.e. up to 0.1 mm), the grave would turn into a hundred-meter rock, ants would increase to the size of the ocean ship, the person would have an increase in 1,700 km. The number of molecules in the amount of substance 1 mole can be determined by the results of experience with the monomolecular layer. Knowing the diameter of the molecule, it is possible to find its volume and the amount of substance 1 mol, which is equal to where P is the density of the liquid. From here, the constant Avogadro is determined. The estimated method is to determine the number of molecules in the amount of substance 1 mol according to the known values \u200b\u200bof the molar mass and the mass of one molecule of the substance. The value of the constant Avogadro, according to modern data, 6,022,169 * 10 23 mol -1. With the estimated method of determining the constant avogadro, you can familiarize students, offering it to calculate in the meanings of the molar masses of different substances. Schoolchildren with a number of horseship, which indicates which number of molecules is contained in a unit of gas volume under normal conditions (it is 2.68799 * 10 -25 m -3). Tenth-graders can independently determine the number of horses for several gases and show that it is in all cases the same thing. The resulting examples can be created by the guys an idea of \u200b\u200bhow large is the number of molecules per unit volume. If the rubber ball is so thin in the rubber balloon that 1,000,000 molecules will go through it every second, it will take about 30 billion. years so that all molecules come out. One of the methods for determining the mass of molecules is based on the experience of perrin, which proceeded from the fact that droplets of the resin in water behave in the same way as molecules in the atmosphere. Perrry calculated the number of droplets in different layers of emulsion, highlighting the layers with a microscope with a thickness of 0.0001 cm. The height on which such droplets are twice as fewer than at the bottom, was equal to H \u003d 3 * 10 -5 m. Mass of one droplet resin It turned out to be equal to m \u003d 8.5 * 10 -18 kg. If our atmosphere only consisted of oxygen molecules, then at a height of H \u003d 5 km, the oxygen density would be two times less than that of the earth's surface. Record the proportion M / M \u003d H / H, where the mass of the oxygen molecule M \u003d 5.1 * 10 -26 kg is found. We offer students to independently calculate the mass of hydrogen molecules, the density of which is two times less than that of the surface of the Earth, at the height H \u003d 80 km. Currently, the molecules are clarified. For example, the oxygen is set to 5.31 * 10 -26 kg, and for hydrogen - 0.33 * 10 -26 kg. When discussing the question of the speeds of movement molecules, they introduce the classical experience of the Stern. When explaining experience, it is advisable to create its model using the device "Rotating disk with accessories". On the edge of the disk in a vertical position, several matches are strengthened, in the center of the disk - the tube with a chute. When the disk is still, the ball, lowered into the tube, rolling around the groove, knocks one of the matches. The disk then leads to rotation at a certain speed recorded along the tachometer. Once again, the broken ball will deviate from the initial direction of movement (relative to the disk) and will someteen a match that is at some distance from the first. Knowing this distance, the radius of the disk and the speed of the ball on the rim of the disk, you can determine the speed of the ball on the radius. After that, it is advisable to consider the essence of the Stern experience and the design of its installation, using the Stern Experience Film Family. Discussing the results of the Stern experience, draw attention to the fact that there is a certain distribution of molecules in speeds, as evidenced by the presence of a strip of sprayed atoms of a certain width, and the thickness of this strip is different. In addition, it is important to note that molecules moving at high speed are settled closer to the site opposite the gap. The greatest number of molecules has the most likely speed. It is necessary to inform students that the theoretically, the law of the distribution of molecules in speeds was opened by J. K. Maxwell. The distribution of molecules in speeds can be modeled on the Halton board. The question of the interaction of molecules schoolchildren has already studied in the VII class, in the X class of knowledge on this issue deepen and expand. It is necessary to emphasize the following points: a) intermolecular interaction has an electromagnetic nature; b) intermolecular interaction is characterized by attraction and repulsion; c) the forces of intermolecular interaction act at distances, not large 2-3 diameters of molecules, and at this distance only the attraction force is noticeable, the repulsion force is almost equal to zero; d) as the distance decreases between the interaction force molecules increase, and the repulsion force increases faster (proportional to M-9) than the force of attraction (proportional to R -7 ). Therefore, with a decrease in the distance between molecules, the attraction force prevails first, then at a certain distance R o the force of attraction is equal to the power of repulsion and the repulsion force prevails with further convergence. All of the above, it is advisable to illustrate a graph of dependence on the distance at first the strength of attraction, the pushing force, and then the resultant force. It is useful to build a chart of potential interaction energy, which can later be used in the consideration of the aggregate states of the substance. The attention of ten-graders appear that the state of a stable equilibrium of interacting particles corresponds to the equality zero of the asylum of the interaction forces and the smallest value of their mutual potential energy. In the solid body, the energy of the interaction of particles (bond energy) is much greater than the kinetic energy of their heat movement, therefore the movement of the particles of the solid is oscillations relative to the nodes of the crystal lattice. If the kinetic energy of the thermal motion of molecules is much more potential energy of their interaction, the movement of molecules is completely disorderly and the substance exists in a gaseous state. If kinetic energy thermal the movements of the particles are comparable to the potential energy of their interaction, the substance is in a liquid state.
Thermal equilibrium.
Temperature. Celsius temperature scale.
Molecular physics and thermodynamics study the properties and behavior of macroscopic systems, i.e. Systems consisting of a huge number of atoms and molecules. Typical systems with which we face in everyday life contain about 1025 atoms.
In the study of such systems, macroscopic values \u200b\u200bimmediately measured by experimental way and characterizing the properties of the entire totality of molecules as a whole. Given the extraordinary complexity of macrosystem, you should start learning from the simplest objects - systems whose condition does not change over time. The state of the macroscopic system in which it can be indefinitely for a long time is called equilibrium (they also speak about it, as a state of thermal equilibrium).
The equilibrium state of the system as a whole can be described using the values \u200b\u200bcalled macroscopic parameters, to the number of which include pressure, volume, etc. Each of the parameters characterizes some system property. So volume V measure properties of the system to occupy one or another area of \u200b\u200bspace; Pressure P - measure The properties of the system resist the external change in its volume.
In the state of thermal equilibrium, macroscopic parameters do not change over time, remain constant.
One of the most important parameters characterizing the equilibrium properties of the macroscopic system is the temperature. We introduce this parameter for which we consider two bodies that can interact and share energy. This type of interaction, which is called thermal, leads to the fact that, as a result of collisions, the molecules in the contact area of \u200b\u200btwo bodies takes place transmission of energy from fast molecules to slow. This means that the energy of atoms in one body decreases, in the other, it increases. The body that loses energy is called more heated, and the body to which the energy passes is less heated. Such a transition of energy continues until the state of thermal equilibrium is established. In the state of thermal equilibrium degree of heated bodies the same. To characterize the degree of heated body, a parameter is introduced, called temperature.
From experience it is known that when the temperature changes, the sizes of bodies, electrical resistance and other properties change. Thus, the temperature can be determined by changing any convenient to measure the physical properties of this substance.
Most often, the fluid property is used to measure the temperature to change the volume when heated and cooling. The device with which the temperature is measured is called a thermometer.
An ordinary liquid thermometer consists of a small glass tank to which a glass tube with a narrow internal channel is attached. The reservoir and part of the tube are filled with mercury or other liquid. The temperature of the medium into which the thermometer is immersed is determined by the top level of mercury in the tube. Divisions on the scale agreed as follows. The number 0 is set in the location of the scale where the level of the liquid column is set when the thermometer is lowered to the melting snow, the number 100 is in the place where the level of the liquid column is set when the thermometer is immersed in the water pair boiling under normal pressure (105 Pa). The distance between these tags is divided into 100 equal parts, called degrees. This temperature scale is created by Celsius. The degree on the Celsius scale is denoted by ° C.
In addition to macroscopic parameters, the parameters of the system are introduced associated with the individual characteristics of the components of its particles, called microscopic. These include the mass of particles, their speed, kinetic energy.
Perfect gas. The main equation of the molecular-kinetic theory of perfect gas.
The theory was created by German physicist R. Clausis in 1957 for a real gas model, which is called the perfect gas. The main signs of the model:
distances between molecules are large compared with their dimensions;
the interaction between molecules is absent at a distance;
in collisions of molecules, there are large repulsive strengths;
collision time is much less than the time of free movement between collisions.
Molecular kinetic theory (MTK) establishes links between the macro and microparameters of the ideal gas. The main MKT equation expresses the connection of gas pressure with the average kinetic energy of the translational movement of molecules. The gas pressure on the vessel walls is the result of numerous molecules blows. With each blow, the wall receives a power pulse, the value of which depends on the velocity of molecules and, therefore, from the energy of their movement. With a huge number of shocks, constant gas pressure on the wall is created. The number of blows depends on the concentration of molecules N. Thus, it can be expected that the gas pressure is associated with the concentration of molecules and with the energy of their movement. We obtain the main MTC equation.
Consider the spherical volume of R radius, in which n molecules of the perfect gas. Consider the movement of one of them. Suppose that the molecule moved straightly with the pulse hit the wall at an angle of W to normal and bounced from it under the same angle, having a pulse. Find the pulse transmitted by the wall by the molecule when hit.
The path that the molecule passes from one strike of the wall to another is equal to the chord of the AB, that is, the value of 2rcossh.
We find the number of blows of the molecule about the wall in one second. It is equal to the ratio of the velocity of the molecule to the path passing the molecule from one collision with the wall to the other.
From the II of Newton's law, it follows that the impulse reported per unit of time by the wall is numerically equal to force, therefore the pressure force acting on the surface of the vessel.
This equation is called the basic equation of the molecular-kinetic theory of ideal gas.
We obtain the connection of pressure with the average kinetic energy of the translational motion of the molecule.
Thus, the pressure of the ideal gas is proportional to the product of the concentration of molecules on the average kinetic energy of the translational motion of the molecule. This statement can be considered another formulation of the main equation of the molecular-kinetic theory of perfect gas.
The Law of Dalton.
Consider the gas consisting of molecules of various substances, which is in volume V. due to the chaotic thermal motion of the molecule of each component of the mixture will be distributed in volume evenly, i.e. Since if the remaining gas components were absent. Due to the permanent collisions of molecules with each other, accompanied by partial exchange between them with pulses and energies, thermal equilibrium is installed in the mixture. All this leads to the fact that the pressure of each of the mixture component will not depend on the presence of the rest.
Then the resulting pressure is determined by the total pressure of all components, i.e. For a mixture of gases, the law of Dalton: the pressure of the mixture of ideal gases is equal to the sum of the partial pressures of the gases incoming in it where k is the number of the gas component in the mixture, PK is its partial pressure, i.e. That is the pressure that the K's gas would have if only one occupied the entire volume occupied by the mixture.
The average quadratic speed of molecules.
From the main equation of molecular-kinetic theory, it is possible to obtain a formula for calculating the average quadratic velocity of molecules.
Any change in the state of the gas is called the thermodynamic process.
The simplest processes in perfect gas are isoprocesses. These are processes in which the mass of gas and one of its status parameters (temperature, pressure or volume) remain constant.
Isoprocess, which flows at a constant temperature, is called isothermal.
Experimentally R. Boylel and E. Mariott It was found that at a constant temperature, the product of gas pressure on the volume for this mass of the gas is the permanent value (Boyle-Mariotta law):
Graphically, this law in the coordinates of the PV is depicted by a line called isotherm.
The isoprocess flowing in perfect gas during which the pressure remains constant, is called isobar.
The dependence of the volume of gas on its temperature at constant pressure was established by L. gay-lousak, which showed that the volume of gas of this mass during constant pressure increases linearly with increasing temperature (Gay-Loussa's law):
V \u003d V0 * (1 + * T), (17)
where V is the volume of gas at a temperature T, ° C; V0 is its volume at 0 ° C.
The value is called the temperature coefficient of volume expansion. For all gases \u003d (1/273 ° C-1). Hence,
V \u003d V0 * (1 + * T). (eighteen)
A graphically, the dependence of the volume on temperature is depicted by a straight line - isobar. At very low temperatures (close to - 273 ° C), the law of gay-lousak is not performed, so the solid line on the graph is replaced by dotted line.
The isoprocess flowing into the gas in which the volume remains constant, is called isohorne.
Studies of the dependence of the pressure of this mass of gas from temperature with a constant volume were first conducted by the French physician Charl. It was found that the gas pressure of this mass with a constant volume increases linearly with increasing temperature (Charles Act):
P \u003d P0 (1+ T). (nineteen)
Here p - gas pressure at a temperature T, ° C; P0 is its pressure at 0 ° C.
The value is called the temperature coefficient of pressure. Its value does not depend on the nature of the gas; For all gases \u003d 1/273 ° C-1. In this way,
P \u003d P0 (1 + * T). (twenty)
The graphic dependence of the pressure on temperature is depicted by a straight line - isochora.
Absolute temperature scale.
If the isochora continue to the area of \u200b\u200bnegative temperatures, then at the point of intersection with the abscissa axis we have
P \u003d P0 (1 + * T) \u003d 0. (21)
Hence the temperature at which the pressure of the perfect gas is applied to zero, T \u003d -273 ° C (more precisely, -273.16 ° C). This temperature is chosen as the beginning of the reference of the thermodynamic temperature scale, which was proposed by the English scientist Kelvin. This temperature is called zero Kelvin (or absolute zero).
The temperature, counted along the thermodynamic scale of temperatures, is denoted by T. It is called thermodynamic temperature. Since the melting point of ice at a normal atmospheric pressure, taken in 0 ° C, is equal to 273.16 K-1, then
T \u003d 273,16 + t. (22)
Klaperon equation.
We obtain another form of equations describing the isobaric and isochhore processes, replacing in equations (18) and (20) the temperature counted on the Celsius scale, thermodynamic temperature:
V \u003d V0 (1 + * T) \u003d V0 () \u003d V0
Designating gas volumes at temperatures T1 and T2, as V1 and V2, write
V1 \u003d V0, V2 \u003d V0.
Dividing the metering of these equality, we get the law gay - Loursak in the form
V1 / V2 \u003d T1 / T2 or \u003d SONST.
Charles and Gay-Loursak laws can be combined into one common law that binds the parameters P, V and T with a constant mass of the gas.
Indeed, suppose that the initial state of the gas at m \u003d const is characterized by the parameters V1, P1, T1, and the final - respectively, V2, P2, T2. Let the transition from the initial state in the final state occurs with the help of two processes: isothermal and isobaric. During the first process, change the pressure from P1 on P2. The volume that gas will take after this transition is denoted by V, then according to the law of Boyle-Mariotta, P1V1 \u003d P2V.
At the second stage, the temperature with T1 to T2 is reduced, and the volume will change from V to V2; Consequently, according to Charles.
The equation of the state of the ideal gas is the Mendeleev-Klapairone equation.
The value of the constant included in equation (28), which is denoted as R, for one mole of any gas is the same, so this constant received the name of the universal gas constant.
We will find the numeric value of R in C, for what we take into account that, as follows from the Avogadro law, one mol of any gas at the same pressure and the same temperature occupies the same volume. In particular, at T0 \u003d 273K and the pressure of P0 \u003d 105 Pa, the volume of one praying of the gas is equal to V0 \u003d 22.4 * 10-І MI. Then r \u003d \u003d 8.31 J / (mol * k).
From equation (29) it is easy to obtain an equation for any mass of gas. Gas mass M will take volume
where M is a mass of 1 mol, M / M is the number of gas moles.
Equation (30) is called the Mendeleev equation - Klapaireron and is the main equation connecting gas parameters into thermal equilibrium. Therefore, it is called the equation of the state of the ideal gas.
Temperature - Measure Middle Kinetic Energy
Comparing the equation of the state of the ideal gas and the basic equation of the kinetic theory of gases recorded for one mole (for this, the number of molecules n take an equal number of Avogadro NA).
The average kinetic energy of the translational motion of the molecule does not depend on its nature and is proportional to the absolute temperature of gas T. It follows that the absolute temperature is a measure of the average kinetic energy of molecules.
The value of R / NA \u003d k in equation (31) was the name of the Boltzmann's constant and is a gas constant, assigned to one molecule:
k \u003d 1.38 * 10-23 J / K-23.
Substituting the value of the average kinetic energy of the translational motion of molecules (31) to the main equation of the molecular-kinetic theory of gases, we obtain another form of the equation of the state of the ideal gas:
The gas pressure is proportional to the product of the number of molecules per unit of volume on its thermodynamic temperature. In the heater from the surface of the wire, flipped with electric shock, silver atoms evaporate. Finding out of the heater through the hole in the vacuum chamber, the steam molecules using the slot system are formed into a narrow beam, directed towards two disks rotating with an angular speed. Discussions are used to sort molecules in speeds. The angle between the slots in the disks. The distance between the disks x does not change during the experiment. In order for the pair molecule to get a particle detector receiver, it must go through the slots in the disks. To do this, the time of passing the molecule moving at a speed V between the discs should be equal to the time of rotation of the slot of the second disk to the angle.
Definition
Atom - The smallest particle of this chemical element. All atoms existing in nature are presented in the periodic system of Mendeleev elements.
Atoms are connected in a molecule due to chemical bonds based on electrical interaction. The number of atoms in the molecule can be different. The molecule may consist of one, of two, three and even several hundred atoms.
Definition
Molecule - the smallest particle of this substance, which has its chemical properties.
Molecular kinetic theory- The doctrine on the structure and properties of a substance based on ideas about the existence of atoms and molecules.
The founder of the molecular-kinetic theory is M.V. Lomonosov (1711-1765), which formulated its main provisions and applied them to explain the various thermal phenomena.
The main provisions of the molecular kinetic theory
The main positions of MKT:
- all bodies in nature consist of the smallest particles (atoms and molecules);
- particles are in a continuous chaotic movement, which is called thermal;
- particles interact with each other: between particles are the forces of attraction and repulsion, which depend on the distance between the particles.
Molecular kinetic theory is confirmed by many phenomena.
Mixing various liquids, dissolving solid bodies in liquids is explained by stirring the molecules of various kinds. In this case, the volume of the mixture may differ from the total volume of the components of it. What speaks of different sizes of molecular compounds.
Definition
Diffusion - The phenomenon of penetration of two or several touching substances in each other.
The most intensively diffusion flows in the gases. The spread of odors is due to diffusion. Diffusion indicates that molecules are in constant chaotic movement. The diffusion phenomenon indicates that there are intervals between molecules, i.e. The substance is discrete.
Definition
Brownian motion - Thermal movement of the smallest microscopic particles suspended in liquid or gas.
This phenomenon for the first time watched the English nerd R. Brown in 1827. Watching a floral pollen into a microscope, weighted in the water, he saw that each particle of pollen performs rapid random movements, moving at a certain distance. As a result of individual displacements, each particle of pollen moved along a zigzag trajectory (Fig. 1, a).
Fig.1. Brownian movement: a) the trajectory of the movement of individual particles suspended in the liquid; b) the transfer of the pulse by molecules of a suspended particle.
Further studies of the Brownian movement in various liquids and with various solid particles showed that this movement becomes more intense than the smaller particle size and the higher the experience of the experience. This movement never stops and does not depend on any external reasons.
R. Brown could not explain the observed phenomenon. The theory of Brownian movement was built by A. Einstein in 1905 and received experimental confirmation in the experiments of French physics J. Pereren (1900-1911).
Liquid molecules that are in constant chaotic movement in a collision with a suspended particle transmit some impulse to it (Fig. 1, b). In the case of a particle of large sizes, the number of molecules flushing on it from all sides, their blows are compensated at each moment, and the particle remains almost fixed. If the particle size is very small, then the blows of molecules are not compensated - on one side, a larger number of molecules may hit it than on the other, as a result of which the particle will come into motion. It is such a movement under the influence of disorderly blows of molecules and make Brownian particles. Although Brownian particles by weight in billions times exceeding the mass of individual molecules and move with very low speeds (compared to the speeds of molecules), all their movement can be observed in a microscope.
Examples of solving problems
Example 1.
Example 2.
Definition 1.
Molecular kinetic theory - This is the doctrine of the structure and properties of a substance based on the presentation of the existence of atoms and molecules, as the smallest particles of chemicals.
The main provisions of the molecular-kinetic theory of the molecule:
- All substances can be in a liquid, solid and gaseous state. They are formed from particles that consist of atoms. Elementary molecules may have a complex structure, that is, to have several atoms in their composition. Molecules and atoms are electrically neutral particles, which in certain conditions acquire an additional electric charge and switch to positive or negative ions.
- Atoms and molecules are moving continuously.
- Particles with electrical nature of power interact with each other.
The main positions of the ICT and their examples were listed above. Between the particles there is a small gravitational impact.
Figure 3. one . one . The trajectory of the Brownian particle.
Definition 2.
Brownian movement of molecules and atoms confirms the existence of the main positions of molecular kinetic theory and explicitly justifies it. This thermal movement of particles occurs with molecules weighted or gas.
An experienced justification of the main provisions of molecular kinetic theory
In 1827, R. Brown discovered this movement, which was due to disorderly blows and movements of molecules. Since the process happened chaotic, then the blows could not balance each other. Hence the conclusion that the speed of the Brownian particle cannot be constant, it is constantly changing, and the direction of direction is depicted in the form of a zigzag shown in Figure 3. one . one .
About the Brownian movement was said by A. Einstein in 1905. His theory found confirmation in the experiments of J. Pereren 1908 - 1911.
Definition 3.
Consequence of Einstein's theory: Square offset< r 2 > The Brownian particle relative to the initial position, averaged in many Brownian particles, is proportional to the observation time t.
Expression< r 2 > \u003d D T explains the diffusion law. By theory, we have that D monotonically increases with increasing temperature. Disorder movement is peeled with diffusion.
Definition 4.
Diffusion - This is the determination of the phenomenon of penetration of two or several contacting substances in each other.
This process occurs fast in heterogeneous gas. Due to the examples of diffusion with different densities, you can get a homogeneous mixture. When in one vessel oxygen O 2 and hydrogen H 2 with a partition, then, when removing it, the gases begin to be mixed, forming a hazardous mixture. The process is possible when there is a rise of hydrogen, and at the bottom of the oxygen.
Interpenetration processes also occur in liquids, but much slower. If you dissolve a solid, sugar, in water, then we obtain a homogeneous solution, which is a visual example of diffusion processes in liquids. Under actual conditions, mixing in liquids and in gases are disguised as fast mixing processes, for example, when convection flows occur.
Diffusion of solid bodies is distinguished by its slow motion. If the surface of the interaction of metals is cleaned, then it can be seen that with a large period of time in each of them, atoms of another metal will appear.
Definition 5.
Diffusion and Brownian movement are considered related phenomena.
With the interconancy of particles of both substances, movement randomly, that is, there is a chaotic thermal movement of molecules.
Forces acting between two molecules depend on the distance between them. Molecules are in their composition positive and negative charges. At long distances, the forces of intermolecular attraction predominate, with small - repulsion forces.
Picture 3 . 1 . 2 Displays the dependence of the resulting force F and the potential energy E p of interaction between molecules from the distance between their centers. At the distance R \u003d R 0 force is integrated into zero. This distance is conditionally adopted as a diameter of the molecule. With R \u003d R 0, the potential energy is minimal.
Definition 6.
To remove two molecules with a distance R 0, it should be reported to E 0, called energy of communication or a depth of potential pit.

Figure 3. one . 2.The power of interaction F. and potential interaction energy E R. Two molecules. F\u003e 0 - repulsion force F.< 0 - force of gravity.
Since molecules have small sizes, then simple monatomic can be no more than 10 - 10 m. Complex can achieve hundreds of times more.
Definition 7.
Disorder chaotic movement of molecules called thermal motion.
As an increase in temperature, the kinetic energy of thermal motion increases. At low temperatures, the average kinetic energy, in most cases, it turns out to be less than the value of the depth of the potential pit E 0. This case shows that the molecules are flowing into a liquid or solid with an average distance between them R 0. If the temperature increases, the average kinetic energy of the molecule exceeds E 0, then they diffuse and form a gaseous substance.
In solids, the molecule moves randomly near fixed centers, that is, the positions of equilibrium. In space, it can be distributed irregularly (in amorphous bodies) or to form ordered volumetric structures (crystalline bodies).
Aggregate states of substances
Freedom of thermal motion of molecules is visible in liquids, as they have no binding to the centers, which allows you to move throughout the volume. This explains its fluidity.
Definition 8.
If molecules are located close, then ordered structures with several molecules can form. This phenomenon was called middle order. Far order Characterized for crystalline bodies.
The distance in the gases between molecules is much larger, therefore the active forces are small, and their movements go along the straight, waiting for the next collision. A value of 10-8 m is an average distance between air molecules under normal conditions. Since the interaction of forces is weak, gases are expanding and can fill out any volume of the vessel. When their interaction is striving for zero, they talk about the representation of the perfect gas.
Kinetic model of perfect gas
In the ICT, the amount of substance is considered a proportional number of particles.
Definition 9.
Mole - This is the amount of substance containing so many particles (molecules), as containing atoms in 0, 012 K carbon C 12. Carbon molecule consists of one atom. It follows that 1 mol of the substance has the same amount of molecules. This number is called permanent Avogadro N A: N A \u003d 6, 02 ċ 1023 m o l - 1.
The formula for determining the amount of substance ν it is written by the ratio n of the number of particles per constant Avogadro N A: ν \u003d n n a.
Definition 10.
Weighing one praying substance They call the molar mass M. It is fixed as a formula M \u003d N A ċ M 0.
The expression of the molar mass is produced in kilograms on the mole (to g / m o l b).
Definition 11.
If the substance has a one atom, then there is a place about the atomic weight of the particle. The unit of atom is 1 12 mass of carbon isotope C 12, called atomic unit of mass and recorded as ( but. eat.): 1 a. e. m. \u003d 1, 66 ċ 10 - 27 to
This value coincides with the mass of proton and neutron.
Definition 12.
The ratio of the mass of the atom or molecule of this substance to 1 12 mass of the carbon atom is called relative mass.
If you notice a mistake in the text, please select it and press Ctrl + Enter
Molecular kinetic theory describes the behavior and properties of a special perfect object called perfect gas. The basis of this physical model is the molecular structure of the substance. The creation of molecular theory is associated with the works of R. Clausius, J. Maxwell, D. Joule and L. Boltzmann.
Perfect Gas.. Molecular kinetic theory of perfect gas built on the following parcels:
atoms and molecules can be considered as material dots in continuous motion;
own volume of gas molecules is negligible compared to the volume of the vessel;
all atoms and molecules are distinguishable, that is, there is a fundamental ability to monitor the movement of each particle;
prior to the collision of gas molecules between them, there are no strengths of interaction, and the impact of molecules between themselves and with the walls of the vessel are assumed to be absolutely elastic;
the movement of each atom or gas molecule is described by the laws of classical mechanics.
Laws obtained for perfect gas can be used in the study of real gases. For this, the experimental models of the ideal gas are created, in which the properties of the real gas are close to the characteristics of the ideal gas (for example, at low pressures and high temperatures).
Laws of perfect gas
Mariotta Law:
for this mass of the gas at a constant temperature, the product of the gas pressure on its volume is the value of the permanency: pV \u003d const , (1.1)
for T. = const. , m \u003d Const. .
The curve depicting the relationship between values r and V., characterizes the properties of the substance at a constant temperature, and is called isotherma is a hyperbole (Fig. 1.1.), And the process flowing at a constant temperature is called isothermal.
Laws Gay Lussa:
The volume of this mass of gas at constant pressure changes the layer temperature
V \u003d V. 0 (1 + t. ) for P \u003d Const. , m \u003d Const. . (1.2)

P. = p. 0 (1 + t. ) for V \u003d cons , m \u003d Const. . (1.3)
In equations (1.2) and (1.3), the temperature is pronounced on the Celsius scale, pressure and volume -
0 c, while
 .
.
The process flowing at constant pressure is called isobaricit can be represented as a linear function (Fig. 1.2.).
The process flowing at a constant volume is called isohorough (Fig. 1.3.).
From equations (1.2) and (1.3) it follows that the isobar and isochora crosses the temperature axis at the point t \u003d.1/ \u003d 273.15 С . If you move the start of the reference to this point, then we turn to the Celvin scale.
Entering in formula (1.2) and (1.3) thermodynamic temperature, the laws of gay-lousak you can give a more convenient view:
V. = V. 0 (1+t.) = = V. 0 = =V. 0 T.;
P. = p. 0 (1+t.) = p. 0 = p. 0 T.;
 for
P \u003d Const, M \u003d Const
;
(1.4)
for
P \u003d Const, M \u003d Const
;
(1.4)
 for V \u003d const, m \u003d const
,
(1.5)
for V \u003d const, m \u003d const
,
(1.5)
where indices 1 and 2 refer to arbitrary states lying on one isobar or isochore .
Act of Avogadro:
moth of any gases at the same temperatures and pressures occupy the same volumes.
Under normal conditions, this volume is equal V. , 0 \u003d 22,4110 -3 m 3 / mole . By definition, in one mole of various substances contain one and the same number of molecules equal permanent Avogadro: N. A. = 6,02210 23 mol -1. .
Law of Dalton:
the pressure of the mixture of different ideal gases is equal to the amount of partial pressures. r 1 , r 2 , r 3 … r n included in it gases:
p \u003d R. 1 + R. 2 + R 3 + ... + R. n. .
Partial pressure – this is the pressure that would produce the gas included in the gas mixture if he occupied the volume equal to the volume of the mixture at the same temperature.
The equation of the state of the ideal gas
(Klapairone Mendeleev equation)
There is a certain connection between temperature, volume and pressure. This relationship can be represented by functional dependence:
f (P, V, T)= 0.
In turn, each of the variables ( p, V, T) It is a function of two other variables. The form of the functional dependence for each phase state of the substance (solid, liquid, gaseous) is found experimentally. This is a very laborious process and the status equation is established only for gases that are in a rarefied state, and in an approximate form - for some compressed gases. For substances that are not gaseous condition, this task has not yet been solved.
French physicist B. Klapairon brought the equation of the state of the ideal gasBy combining the laws of Boyle Mariotta, Gay Loursak, Charles:
 . (1.6)
. (1.6)
Expression (1.6) and there is an equation of Klapaireron, where IN- Gas constant. It is different for different gases.
DI. Mendeleev combined the Klapairone equation with the Avogadro law, taken equation (1.6) to one pray and using a molar volume V. . According to Avogadro's law, with the same r and T. Moth of all gases occupy the same molar volume V. .
.
Therefore, constant IN It will be the same for all perfect gases. This constant is usually indicated. R. and equal R.=
8,31
 .
.
Clapierone Mendeleev equation it has the following form:
P. V. . = R T..
From equation (1.7), for one praying gas, you can go to clapareron Mendeleev equation for arbitrary gas mass:
 , (1.7)
, (1.7)
where
–
molar mass
(Mass of one praying substance, kg / mol); M.
gas weight;
 Number of substances .
Number of substances .
More often use another form of an ideal gas equation, introducing Permanent Boltzmanna:  .
.
Then the equation (1.7) looks like this:
 ,
(1.8)
,
(1.8)
where
 –
concentration of molecules (number of molecules per unit volume). From this expression it follows that the pressure of the ideal gas is directly proportional to the concentration of its molecules or gas density. With the same temperatures and pressures, all gases contain a single number of molecules per unit. The number of molecules contained in 1 m 3 under normal conditions is called
number of horsemen:
–
concentration of molecules (number of molecules per unit volume). From this expression it follows that the pressure of the ideal gas is directly proportional to the concentration of its molecules or gas density. With the same temperatures and pressures, all gases contain a single number of molecules per unit. The number of molecules contained in 1 m 3 under normal conditions is called
number of horsemen:
N. L. = 2.68 10 25 M -3.
The main equation of molecular kinetic
theories of ideal gases
The most important task the kinetic theory is gas in the theoretical calculation of the pressure of the perfect gas based on molecular kinetic representations. The main equation of the molecular-kinetic theory of ideal gases is output using statistical methods.
It is assumed that gas molecules are moving chaotic, the number of mutual collisions between the gas molecules is negligible compared to the number of shocks about the vessel wall, and these collisions are absolutely elastic. On the wall of the vessel, some elementary platform is distinguished S. And the pressure is calculated, which will have gas molecules on this platform.
It is necessary to take into account that actually molecules can move to the site at different angles and may have different speeds that, moreover, with each collision can change. In theoretical calculations, the chaotic movements of molecules is idealized, they are replaced by a movement along three mutually perpendicular directions.
If we consider the vessel in the form of a cube in which it is randomly moving N.gas molecules in six directions, it is easy to notic that 1/3 of all molecules are moving along each of them, and half of them (i.e. 1/6 of all molecules) moves one way, and the second half is half (also 1/6) in the opposite. With each collision, a separate molecule moving perpendicular to the platform, reflecting, transmits it with a pulse, with its number of movement (pulse) changes by magnitude
R 1 =m. 0 v. – (– m. 0 v.) = 2 m. 0 v..
The number of blows of molecules moving in a given direction, the site will be equal to: N. = 1/6 n. S.v.t.. When a collision with a platform, these molecules will be transferred to it impulse
P.= N. P. 1 =2 m. 0 v. N.S.v.t \u003d M. 0 v. 2 n.S.t.,
where n. - concentration of molecules. Then the pressure that the gas is provided by the vessel wall will be equal to:
P \u003d.  =
n M. 0
v. 2
.
(1.9)
=
n M. 0
v. 2
.
(1.9)
However, gas molecules are moving at different speeds: v. 1 , v. 2 , …,v. n. , Therefore, speeds must be averaged. The sum of the squares of the speed of movement of the gas molecules, divided by their number, determines the root-mean-square speed:
 .
.
Equation (1.9) type:
 (1.10)
(1.10)
expression (1.10) called the main equation of molecular kinetic theoryideal gases.
Considering that  We will get:
We will get:
p V \u003d n  \u003d E.,
(1.11)
\u003d E.,
(1.11)
where E. - the total kinetic energy of the progressive movement of all gas molecules. Consequently, the gas pressure is directly proportional to the kinetic energy of the translational movement of the gas molecules.
For one praying gas m \u003d., and the Klapairone Mendeleev equation has the following form:
P V. . \u003d R T.,
and since from (1.11) it follows that p V. . = v. kv. 2, we get:
RT \u003d. v. kv. 2. .
Hence the average quadratic speed of gas molecules is equal
v.
kv.
=
 =
= =
= ,
,
where k. = R./ N. A. \u003d 1,38 €10 -23 J / K - permanent Boltzmann. From here you can find the average quadratic speed of oxygen molecules at room temperature - 480 m / s, hydrogen - 1900 m / s.
Molecular Kinetic Meaning Temperature
The temperature is the quantitative measure of the "heatedness" of the body. To clarify the physical meaning of absolute thermodynamic temperature T. Comparison the basic equation of molecular-kinetic theory of gases (1.14) with the Klapaireron-Mendeleev equation p.V. = R T.
Equating the right parts of these equations, we find the average value of the kinetic energy 0 of one molecule ( \u003d N./N. A. K.= R./N. A.):
 .
.
From this equation, the most important output of the molecular-kinetic theory is followed: the average kinetic energy of the translational movement of one ideal gas molecule depends only on temperature, while it is directly proportional to the thermodynamic temperature. Thus, the thermodynamic scale of temperatures acquires a direct physical meaning: T. \u003d 0 kinetic energy of ideal gas molecules is zero. Consequently, based on this theory, the translational movement of gas molecules will stop and its pressure will become equal to zero.
The theory of the equilibrium properties of the perfect gas
The number of degrees of freedom of molecules. The molecular-kinetic theory of ideal gases leads to a very important consequence: gas molecules make an erratic movement, with the average kinetic energy of the translational movement of the molecule, is determined exclusively to the temperature.
The kinetic energy of the movement of molecules is not exhausted kinetic energy of translational movement: It also develops from kinetic energi rotation and oscillations molecules. In order to calculate the energy that goes on all types of movement of molecules, it is necessary to define the number of degrees of freedom.
Under the number of degrees of freedom (i.) The body is meant the number of independent coordinates that must be entered to determine the position of the body in space.
N.  for example, the material point has three degrees of freedom, since its position in space is determined by three coordinates: x, W.and z.. Consequently, a one-cattle molecule possesses three degrees of freedom of translational movement.
for example, the material point has three degrees of freedom, since its position in space is determined by three coordinates: x, W.and z.. Consequently, a one-cattle molecule possesses three degrees of freedom of translational movement.
D.  vochant molecule has 5 degrees of freedom (Fig. 1.4): 3 degrees of freedom of translational movement and 2 degrees of freedom of rotational motion.
vochant molecule has 5 degrees of freedom (Fig. 1.4): 3 degrees of freedom of translational movement and 2 degrees of freedom of rotational motion.
Molecules of three or more atoms have 6 degrees of freedom: 3 degrees of freedom of translational movement and 3 degrees of freedom of rotational motion (Fig. 1.5).
Each gas molecule has a certain number of degrees of freedom, three of which correspond to its translational movement.
Energy Equality Regulations
in degrees of freedom
The main prerequisite of the molecular-kinetic gases theory is the assumption of the complete mess of the movement of molecules. This also applies to oscillatory, and to rotational motion, and not just a progressive. It is believed that all directions of movement of molecules in Gaza are equally even. Therefore, it can be assumed that for each degree of freedom of the molecule, one and the same amount of energy accounts for an average - this is the provision on the equilibrium of energy in the degrees of freedom. The energy coming on one degree of freedom of the molecule is equal to:
 . (1.12)
. (1.12)
If the molecule has I. degrees of freedom, then on each degree of freedom accounts for an average:
 .
(1.13)
.
(1.13)
Internal energy of perfect gas
If you attribute the full supply of the internal gas energy to one pray, then we get its meaning, multiplying for the number of Avogadro:
 .
(1.14)
.
(1.14)
It follows that the internal energy of one mole of the ideal gas depends only on temperature and number of degrees of freedom of gas molecules.
distribution Maxwell and Boltzmann
The distribution of ideal gas molecules in the speeds and energy of thermal motion (distribution of Maxwell). At a constant gas temperature, all directions of movement of molecules are assumed to be equivalent. In this case, the average quadratic velocity of each molecule remains constant and equal to
 .
.
This is due to the fact that in the ideal gas, which is in a state of equilibrium, establishes some stationary, non-changing distribution of molecules in speeds. This distribution obeys a certain statistical law, which theoretically brought J. Maxwell. Maxwell law is described by the function
 ,
,
that is the function f.(v.) Determines the relative number of molecules  whose velocities lie in the interval from v.
before v. + D.v.. Applying probability theory methods, Maxwell found the law of the distribution of the molecules of the ideal gas at speeds:
whose velocities lie in the interval from v.
before v. + D.v.. Applying probability theory methods, Maxwell found the law of the distribution of the molecules of the ideal gas at speeds:
 . (1.15)
. (1.15)
The distribution function in graphical form is presented in Fig. 1.6. The area, limited distribution curve and the abscissa axis is equal to one. This means that the function f.(v.) Satisfies the normalization condition:
 .
.
FROM  crough in which the function of the distribution of the molecules of the ideal gas at speeds F.(v.) maximum called most likely
speed
v. B. .
crough in which the function of the distribution of the molecules of the ideal gas at speeds F.(v.) maximum called most likely
speed
v. B. .
Values v. = 0 and v. = correspond to the minimizes of expression (1.15). The most likely speed can be found by indigning expression (1.23) and equating it to zero:
 =
= =
1,41
=
1,41
With increasing temperature, the maximum function will shift to the right (Fig. 1.6), that is, with increasing temperature, the most likely rate increases, however, the limited curve area remains unchanged. It should be noted that in the gases and at low temperatures there is always a small amount of molecules that are moving with high speeds. The presence of such "hot" molecules is of great importance when many processes occur.
Average arithmetic speed Molecules are determined by the formula
 .
.
Medium quadratic speed
 =
1,73
=
1,73 .
.
The ratio of these velocities does not depend on the temperature or on the type of gas.
Function of the distribution of molecules by heat movement energies. This function can be obtained by substituting in the equation of distribution of molecules (1.15) instead of a speed of kinetic energy:
 .
.
Integrating the expression on the values \u200b\u200bof energy from  before
before 
 , get middle kinetic energy Molecules of perfect gas:
, get middle kinetic energy Molecules of perfect gas:
 .
.
Barometric formula. Boltzmann distribution. When the main equation of molecular-kinetic theory of gases and the distribution of Maxwell molecules was assumed that external forces do not act on the molecules of ideal gas, so molecules are evenly distributed throughout the volume. However, any gas molecules are in the field of land. In the conclusion of the law of pressure dependence on the height, it is assumed that the field is uniform, the temperature is constant and the mass of all molecules is the same:
 . (1.16)
. (1.16)
Expression (1.16) called barometric formula. It allows you to find atmospheric pressure depending on the height or, measuring the pressure, you can find the height. As h. 1 - This is a height above sea level, where pressure is considered normal, the expression can be modified:
 .
.
The barometric formula can be converted if you use the expression p \u003d nkt.:
 ,
,
g.  de n.
–
concentration of molecules at height h.,
m. 0
gH.= P–
the potential energy of the molecule in the field of gravity. At a constant temperature, the gas density is greater where the potential energy of the molecule. A graphically of the decrease in the number of particles per unit volume with a height looks, as shown in Fig. 1.7.
de n.
–
concentration of molecules at height h.,
m. 0
gH.= P–
the potential energy of the molecule in the field of gravity. At a constant temperature, the gas density is greater where the potential energy of the molecule. A graphically of the decrease in the number of particles per unit volume with a height looks, as shown in Fig. 1.7.
For an arbitrary external potential field, we write the following overall expression
 ,
,

 Fig. 3.3.
Fig. 3.3.